By utilizing the Investigator SFC System, the typical workflow for small scale chiral purification, from method development, method optimization, and scale-up, to post-purification analysis, can be accomplished on a single platform. This system is ideal for laboratories routinely performing chiral analysis and small scale purification up to tens of milligrams.
By utilizing the Waters Investigator SFC System, the typical workflow for small scale chiral purification, from method development, method optimization, and scale-up, to post-purification analysis, can be accomplished on a single platform. The Investigator SFC System is ideal for industries facing escalating pressure for improved productivity while adhering to stricter deadlines and budgets.
Supercritical fluid chromatography (SFC) purification often entails method development to identify optimal stationary phases and mobile phases, a loading study to determine the maximum mass and volume loading, preparative chromatography, and post-purification analysis of the collected fractions for quality control (QC). Typically, the process is accomplished by using both analytical and preparative instrumentation; however, the Investigator SFC System provides method development, purification for up to tens of milligrams, and post-purification analysis on a single platform. Combined with the inherent advantages of SFC, this system is ideal for pharmaceutical companies facing escalating pressure to research, develop, and release new drugs to the marketplace while adhering to stricter deadlines and budgets.
One of the key application areas of SFC is chiral separations. In recent years, there are increasingly stringent guidelines on stereoisomers from the FDA, EMEA, and ICH. For example, the FDA policy states that when developing stereoisomeric drugs, each isomer should be evaluated individually.1 Because of these new guidelines, there have been increased demands in the pharmaceutical industry to generate enantiomerically pure compounds before undertaking pharmacokinetic, metabolic, physiological, and toxicological evaluations.
In general, SFC is deemed by many a greener and more cost-effective preparative chromatographic technique. Due to the higher diffusivity and lower viscosity of supercritical fluid, SFC often provides a three- to eight-fold faster separation than normal phase LC, resulting in a measurable increase in productivity. Compared to normal phase LC, SFC also offers unique selectivity, less organic solvent consumption and waste removal, smaller collection volume, and faster postpurification dry-down time.
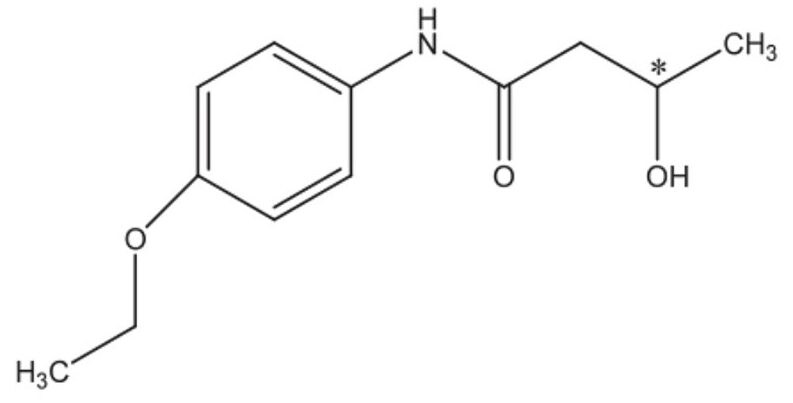
Herein, we use bucetin as a model compound to demonstrate the chiral SFC purification workflow on the Investigator SFC System. Bucetin, a chiral pharmaceutical compound displayed in Figure 1, is used as an analgesic when combined with other drugs. Method development, gradient-isocratic conversion, loading study, purification using stacked injections, and post-purification analysis are illustrated.
All experiments were performed on the Investigator SFC System. The system consists of the following: Fluid Delivery Module (FDM), Automated Back Pressure Regulator (ABPR), Alias Autosampler, 10-port Analytical-2-Prep Column Oven, 2998 PDA Detector, make-up pump, and six-position Fraction Collection Module. The system is controlled by SuperChrom Software.
A racemic (R, S)-bucetin standard was obtained from Sigma-Aldrich (St. Louis, MO). The structure of (R, S)-bucetin is shown in Figure 1. The sample was dissolved in methanol at 1 mg/mL and 10 mg/mL, for analytical and preparative injections, respectively.
Six chiral columns (4.6 x 250 mm, 5 μm): AD-H, OD-H, (R, R)-Whelk-O1, AS-H, OJ-H, and IC, were all purchased from commercial sources. Key parameters for the screening and isocratic experiments were as follows:
|
|
|
Gradient |
Isocratic |
|
|
Flow rate: |
|
3 mL/min |
3 mL/min |
|
|
Co-solvent: |
Methanol |
Methanol |
||
|
Back pressure: |
120 bar |
120 bar |
||
|
Temp.: |
|
40 °C |
40 °C |
|
|
PDA: |
|
220 to 300 nm |
220 to 300 nm |
|
|
Injection vol.: |
|
5 μL |
5 μL |
|
|
Co-solvent: |
Time |
Gradient |
Isocratic |
|
|
|
Initial |
0.05 |
0.2 |
|
|
|
5 min: |
5% to 40% |
|
|
|
|
7 min: |
0.4 |
|
|
|
|
8 min: |
40% to 5% |
|
|
|
|
10 min: |
0.05 |
|
A semi-preparative AD-H column (10 x 250 mm, 5 μm) was used for all preparative chromatography. The key experimental parameters were as follows:
|
Flow rate: |
14 mL/min |
||
|
Make up: |
3 mL/min |
||
|
Co-solvent: |
Methanol |
||
|
Isocratic:: |
20% co-solvent |
||
|
Back pressure: |
120 bar |
||
|
Temp.: |
40 °C |
||
|
PDA: |
220 to 300 nm |
||
|
Injection vol.: |
Varying from 10 to 100 μL |
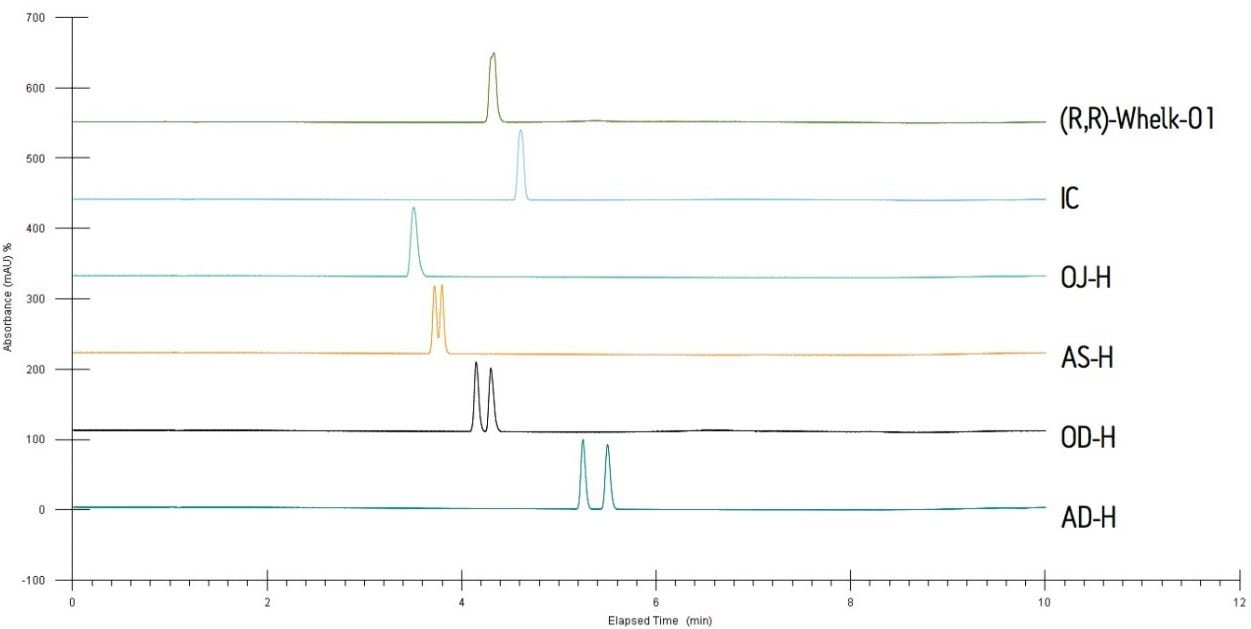
SFC purification often starts with the screening of multiple chiral stationary phases (CSPs) and mobile phases, also referred to as “scouting.” Figure 2 shows the SFC chromatograms with six different CSPs using a generic gradient. The results indicated that the AD-H, OD-H, and AS-H columns were capable of separating the bucetin enantiomers. The AD-H column, however, yielded the highest resolution between the enantiomeric pair. It was, therefore, chosen for the ensuing experiments.
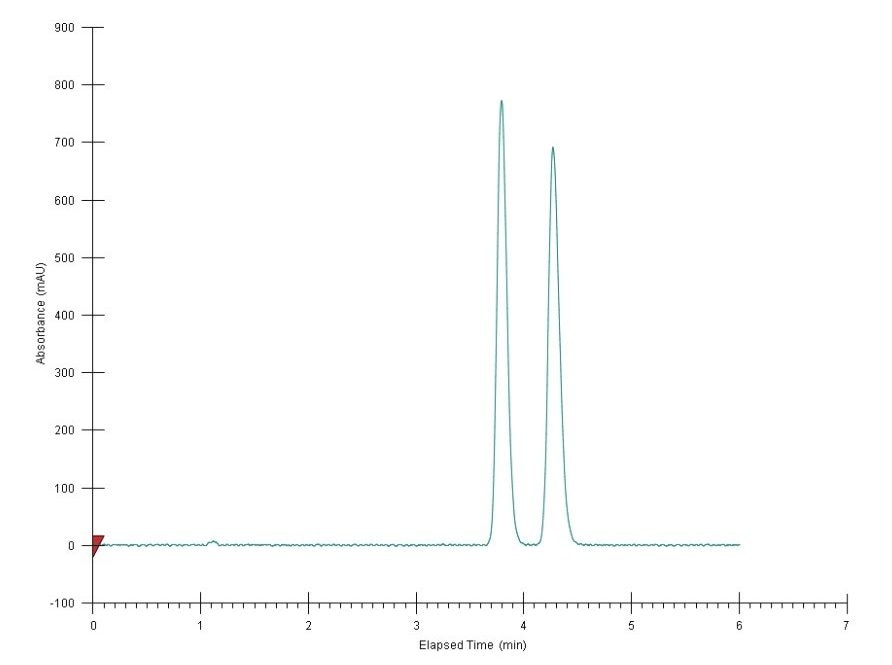
Next, the gradient method was converted to an isocratic method, with the resulting chromatogram shown in Figure 3. Isocratic conditions can alleviate the stress on the pumps associated with gradient ramping on a large-scale chromatography system, leading to more reproducible chromatography which is critical for collection. Furthermore, isocratic conditions are a prerequisite for employing stacked injections in preparative SFC for high productivity. It is noteworthy that both resolution and run time, the two competing factors for overall productivity, should be carefully considered when developing an appropriate isocratic method. Typically, a high percentage of co-solvent leads to a shorter run time but reduced resolution. The resolution further deteriorates in preparative chromatography, due to large mass/volume injections. A successful isocratic method often involves a compromise between run time and resolution, and is determined experimentally. In this case, isocratic at 20% co-solvent appeared to be a sensible choice. The flow rate scale-up follows the general equation: F1/F2 = d21/d22. For the 10-mm I.D. column used in this study, the flow rate was: (10/4.6)2 x 3 = 14 mL/min. All the ensuing preparative chromatography was run at 14 mL/min with 20% co-solvent.
A loading study was then conducted on a 10-mm I.D. AD-H column to determine the maximum loading for preparative runs. The results are shown in Figure 4. The injection volume varied from 10 to 100 μL. At 90 μL, the bucetin enantiomers were still separated, but no longer baseline resolved. The maximum effective loading was 80 μL or 800 μg in mass per injection.
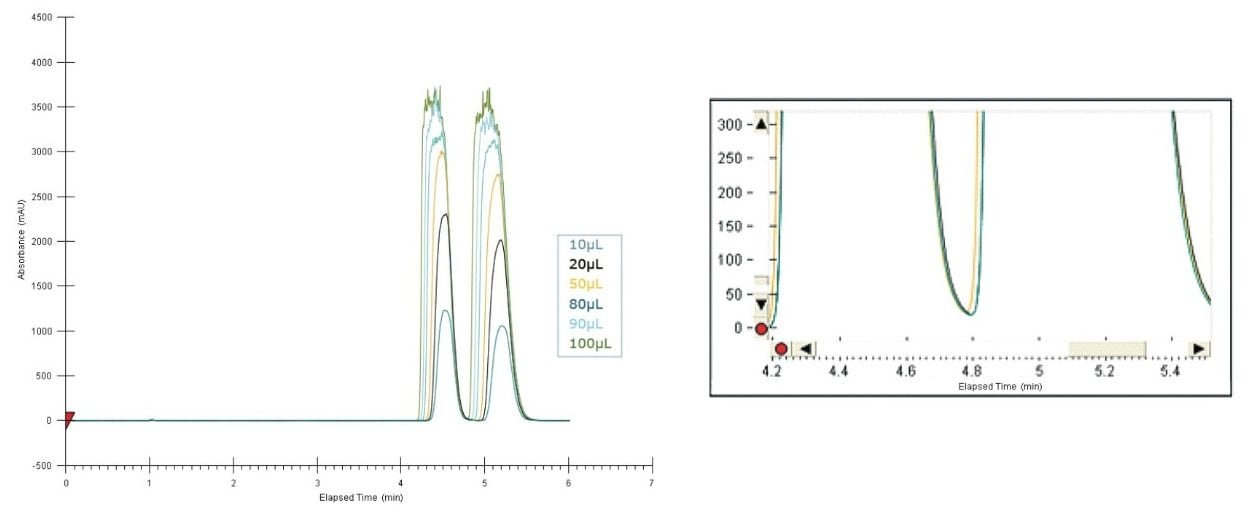
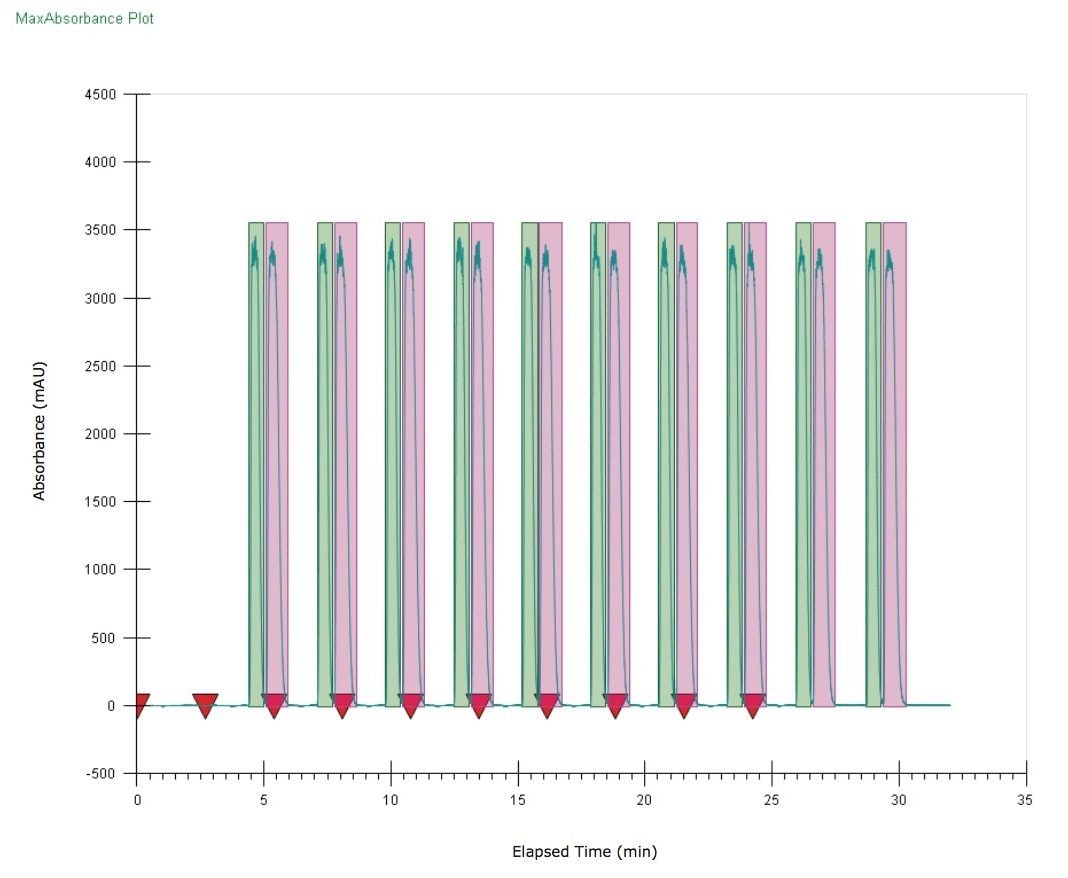
Figure 5 shows the SFC chromatograms of (R, S)-bucetin (10 mg/mL) from a sequence of 10 injections using stacked injections. Each injection was 80 μL. A total of 8 mg (4 mg for each enantiomer) was purified in 30 min and the total solvent use was 30 min x 14 mL/min = 420 mL. If using a regular injection scheme, the total run time will be 60 min and the total solvent use will be 60 min x 14 mL/min = 840 mL. Stacked injection is an effective means to improve productivity without compromising chromatographic efficiency. Performed under isocratic conditions, injections are made during the course of chromatography so that the first peak from a subsequent injection elutes off the column adjacent to the last peak of the preceding injection. The chromatographic space is efficiently populated by peaks from consecutive injections, resulting in noticeable savings of both time and solvents.
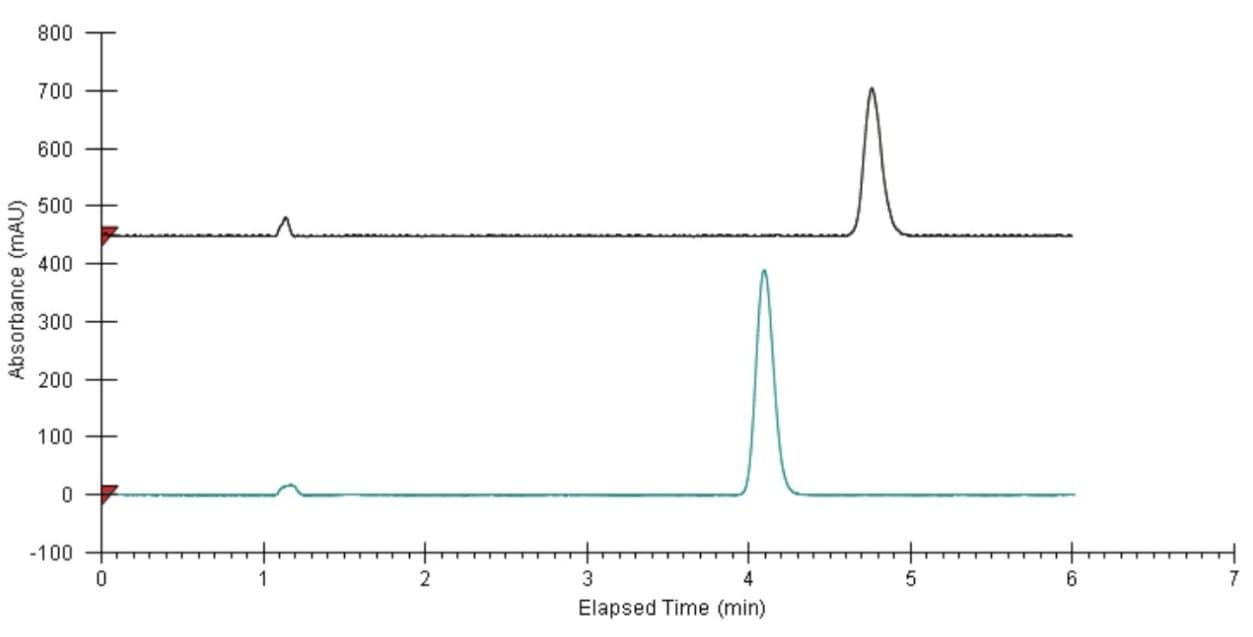
Finally, the two fractions were re-analyzed using the isocratic analytical method and the resulting chromatograms are shown in Figure 6. Both fractions showed 100% purity. No recovery measurement was attempted.
Using racemic bucetin as a model compound, the complete workflow of chiral method development, scale-up, and small scale purification has been successfully demonstrated with the Investigator SFC System. Chiral screening using SFC quickly determined the optimal column for the separation of R- and S-bucetin. An isocratic method was developed to take advantage of stacked injections in purification and the method was easily scaled up following the same principle as LC. Stacked injections allowed for the fast purification of a total of 8 mg of bucetin racemate in 30 minutes, yielding high purity fractions of the bucetin enantiomers consuming a smaller volume of organic solvents than would be required without stacked injections. The Investigator SFC System is a versatile, single SFC platform ideal for laboratories routinely performing chiral analysis and small scale purification up to tens of milligrams.
720004141, October 2011