Using Charge Detection Mass Spectrometry with an Electrostatic Linear Ion Trap and Heated Inlet for the Analysis of Protein Complexes
Rosie Upton, Anisha Haris, Jakub Ujma, Kevin Giles, David Bruton, Keith Richardson, Ying Qing Yu, Bradley Davis, Madison Turner, Siavash Vahidi
Waters Corporation, United States
Published on October 13, 2025
Abstract
Large and heterogeneous native protein complexes challenge conventional mass spectrometry (MS) due to overlapping signals and unresolved charge states. Charge detection mass spectrometry (CDMS) using an electrostatic linear ion trap (ELIT), directly measures the mass of individual ions and overcomes these limitations. Here, it is shown how a novel heated inlet enhances structural insights by activating and dissociating thermally sensitive protein complexes. Applied to the proteasome system, the Waters™ Xevo™ CDMS revealed that Bpa (bacterial proteasome activator), a dodecameric proteasome regulatory particle, binds two copies of the HspR (heat shock repressor) substrate with minimal stoichiometric heterogeneity. Heat-induced substrate dissociation and asymmetric charge partitioning of the intact 20S proteasome core particle showed excellent agreement between the experimental and theoretical mass, and exhibited thermally triggered ejection of α-subunit above 250 °C. These findings highlight CDMS, along with thermal activation, as a powerful method for probing the stoichiometry of high-mass noncovalent complexes and advances an understanding of disease-relevant biomolecular assemblies.
Benefits
- Precise mass characterization: Xevo CDMS enables accurate measurement of large, heterogeneous protein complexes by directly determining both m/z and charge of individual ions.
- Enhanced structural insights: The heated inlet allows observation of heat-induced dissociation and charge reduction, revealing conformational changes and stability of protein assemblies.
Introduction
Deciphering the structural features and interactions of native protein complexes is key to understanding their biological function and advancing therapeutic strategies. However, their large size and inherent heterogeneity present considerable challenges to conventional analytical methods. With time-of-flight (ToF) MS for example, low detection limits for high mass-to-charge (m/z) species coupled with broad peaks due to adduction and heterogeneity result in unresolvable charge states. Charge resolution is required for robust spectral deconvolution and accurate mass measurement.1 Overcome these barriers with CDMS, employing an ELIT, which enables direct mass measurement of individual ions through the simultaneous determination of their m/z and charge (z). This approach resolves overlapping and ambiguous charge states obtained using conventional high-resolution mass spectrometry (HRMS), allowing precise characterization of heterogeneous assemblies.
Here, a novel heated inlet is introduced that activates native protein complexes for CDMS analysis, thereby facilitating enhanced structural interrogation. This innovation broadens the ability to investigate complex biological systems with greater resolution and provides valuable orthogonal data to guide decision-making around downstream studies, such as cryo-EM, which are resource-intensive and time-consuming.
This methodology is applied to Mycobacterium tuberculosis (Mtb), which harbors a rare prokaryotic proteasome system vital for immune evasion. Central to this system is the 20S core particle (CP), a 720 kDa protease formed through the stacking of four homoheptameric rings in an α7-β7-β7-α7 configuration. Enzymatic function is carried out by β7-rings, however, access to the catalytic sites of the 20S CP is tightly regulated to prevent off-target degradation. The α7-rings form gated pores to restrict access to the central degradation chamber, therefore requiring regulatory particles to facilitate access.2 This is handled in part by Bpa, an ATP-independent regulatory particle that forms a homododecameric ring (228 kDa). Bpa is known to bind substrate proteins such as HspR and translocates them to the 20S CP for degradation.3
Despite biochemical evidence of Bpa’s function, the complex stoichiometry, oligomeric heterogeneity, and detailed structure of the Bpa:HspR complex remain unresolved. Here, a recently commercialized CDMS instrument is used with thermal activation to address these gaps, enabling interrogation of complex assembly and dissociation dynamics. Notably, inlet heating also allowed us to probe the stoichiometry of the 20S CP itself, revealing temperature-dependent subunit losses and offering insight into its structural integrity and thermal stability. This dual capability positions CDMS as a powerful tool for analyzing noncovalent complexes implicated in human health.
Experimental
Sample Description
Bpa and 20S were produced recombinantly in E. coli and purified at the University of Guelph. All samples were buffer exchanged into ammonium acetate solution using either 10 or 100 kDa molecular weight cut-off filters (Amicon), for Bpa and 20S, respectively.
Method Conditions
A nano-electrospray ionization (nESI) source operated in positive-ion mode was used to generate ions. Mass analysis was performed using a prototype ELIT-based CDMS system (Figure 1) with 500 ms trapping time. For the thermal activation experiments, the heated inlet was operated at 300 °C. Signal processing and data visualization was performed using software developed in-house. Detected time-domain signals were Fourier transformed; the measured frequency and the magnitude correspond to an individual ion’s m/z and z, respectively, enabling direct calculation of mass values. Data for individual ions were combined and binned as histograms to generate m/z, charge, and mass spectra as well as two-dimensional heatmaps.
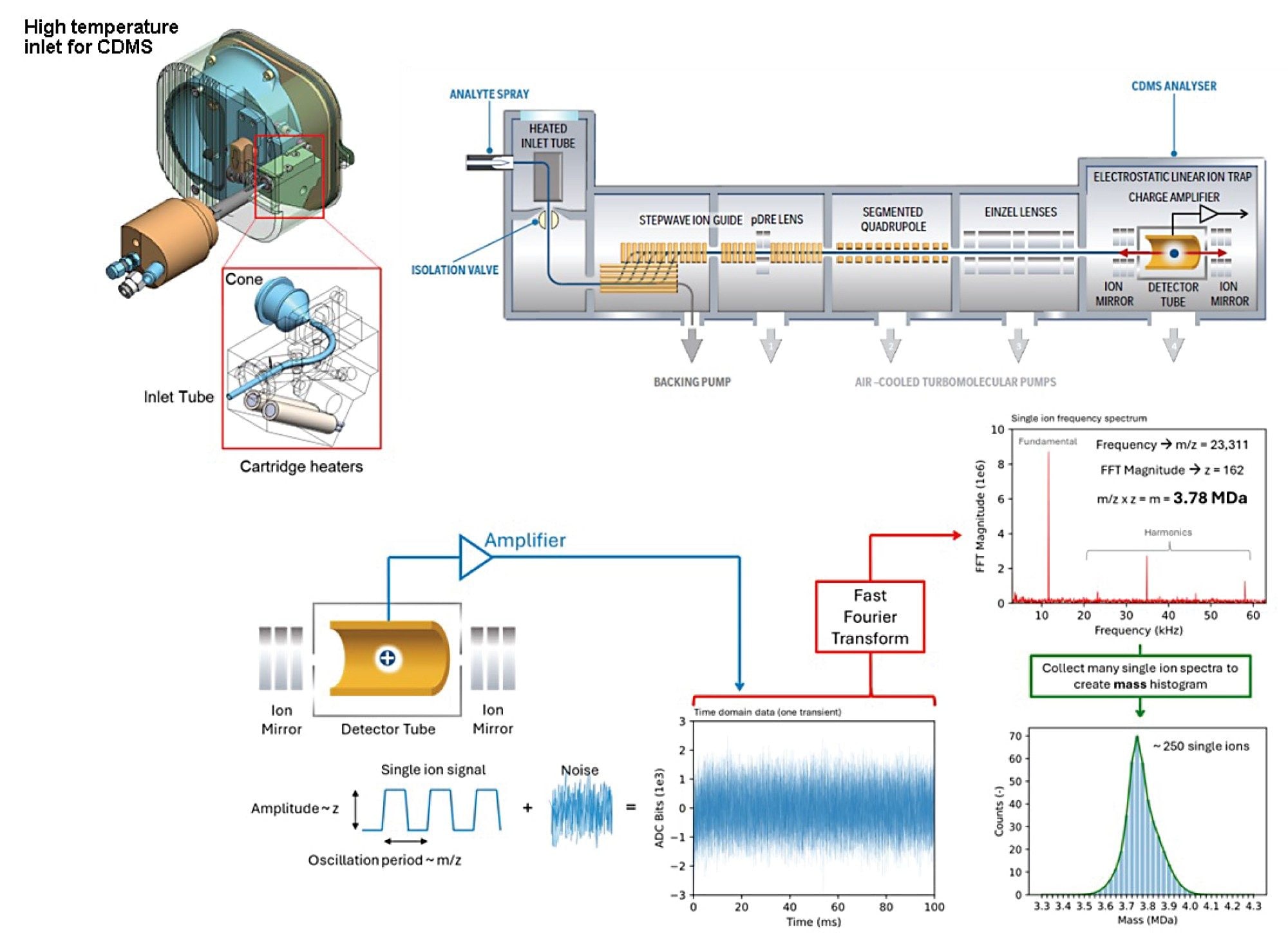
Results and Discussion
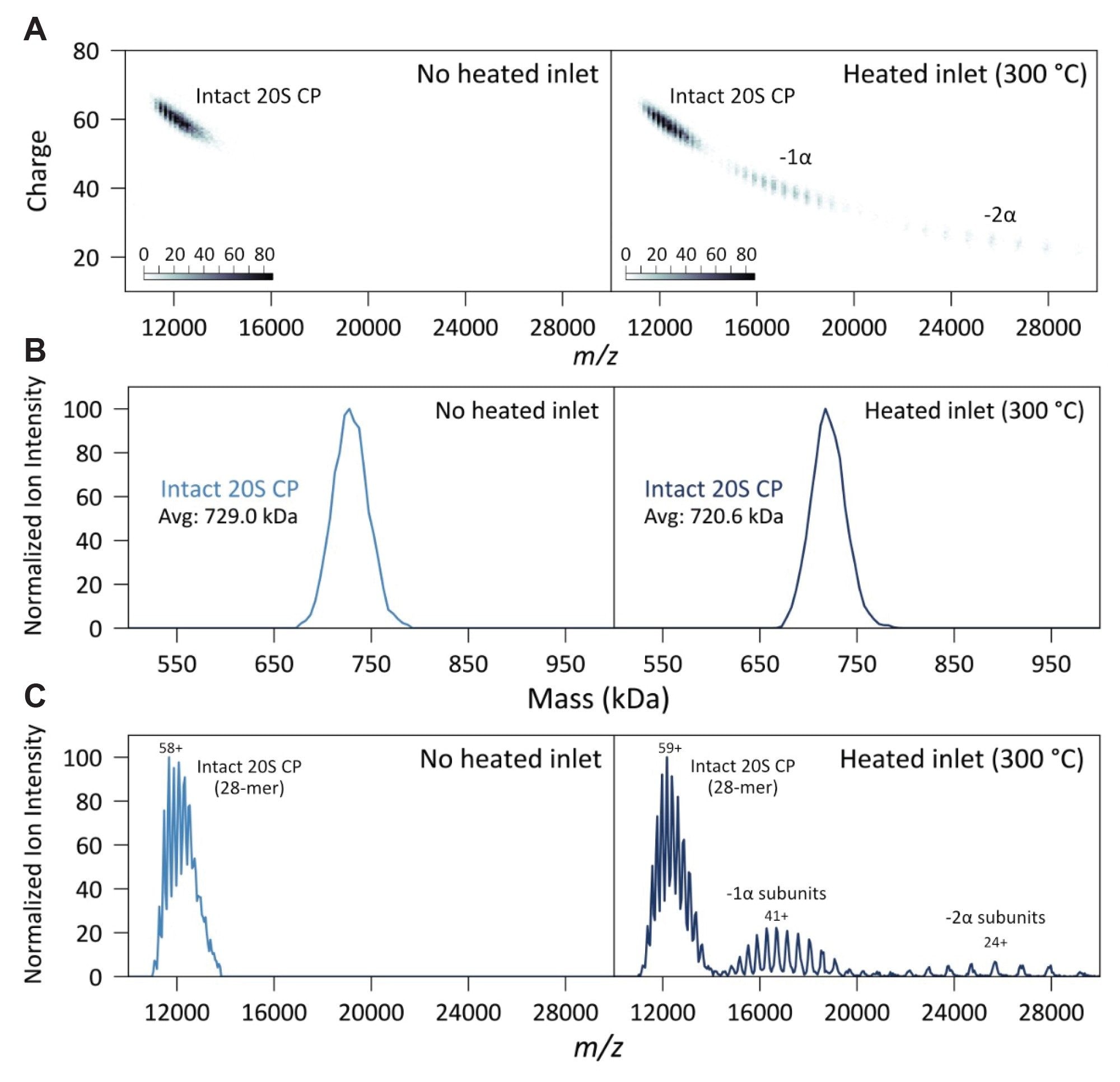
CDMS was first used to analyze the well-characterized 28-subunit 20S CP as a model system. The measured intact mass of the native complex (729 kDa) closely matched the theoretical value (720 kDa), demonstrating the accuracy of CDMS for large noncovalent assemblies (Figure 2). Upon heating the inlet to 300 °C, a clear loss of charge was observed in a subset of species, consistent with asymmetric charge partitioning. This resulted in a higher m/z envelope at 16,000–20,000 with resolved charge states corresponding to the loss of a single α-subunit. A secondary, low intensity m/z envelope was also observed around m/z 23,000-27,000 consistent with the loss of a second α-subunit. Charge vs. m/z heat maps for the native and heated-inlet conditions further illustrate subunit loss and the associated charge reduction. The m/z density plots and the mass spectra for the native and heated-inlet 20S CP data highlight the subunit loss and corresponding charge reduction (Figures 2A and 2C, respectively). The associated mass loss of the alpha subunits is demonstrated in Figure 2B. Notably, thermal activation also promoted the release of nonspecific adducts that remained bound to the intact 20S CP under native conditions. Their removal resulted in an excellent match between the measured and theoretical masses, highlighting the utility of inlet heating for improving mass accuracy in heterogeneous complexes.
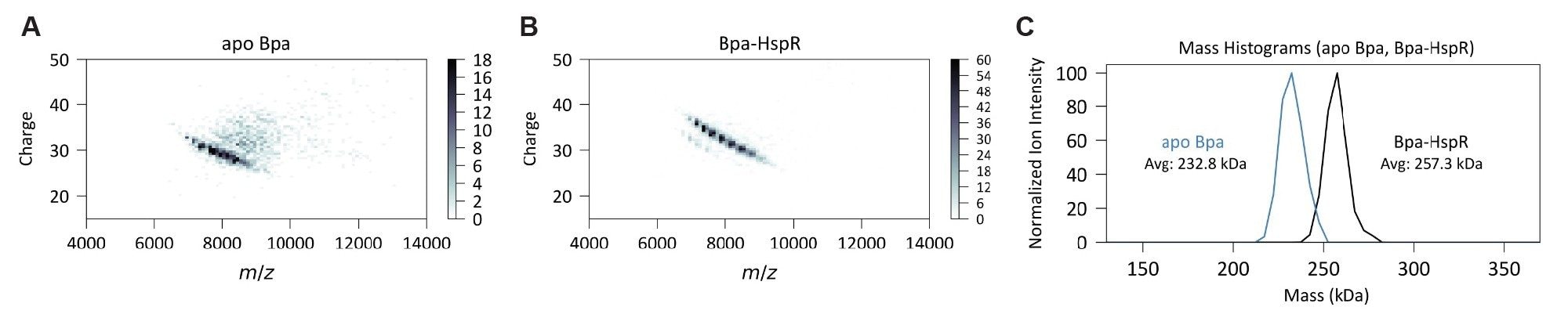
Next, an unbound (apo) and HspR-bound Bpa by ELIT-CDMS with and without inlet heating was analyzed to gain insight into the composition of the complex, whose precise stoichiometry remains unknown. The resulting spectra established dodecameric Bpa bound to two copies of HspR, with minimal heterogeneity in the number of HspR subunits bound. Apo Bpa under ambient conditions had a measured mass of 232.8 kDa, compared with the theoretical mass of 227.3 kDa. Bpa-HspR had an observed mass of 257.3 kDa, compared to the theoretical mass of 255.6 kDa. This observed mass difference of 24.5 kDa equates to 1.73 molecules of HspR being bound to Bpa (Figure 3). With HspR bound, there was decreased heterogeneity and improved charge state resolution (Figure 3B).
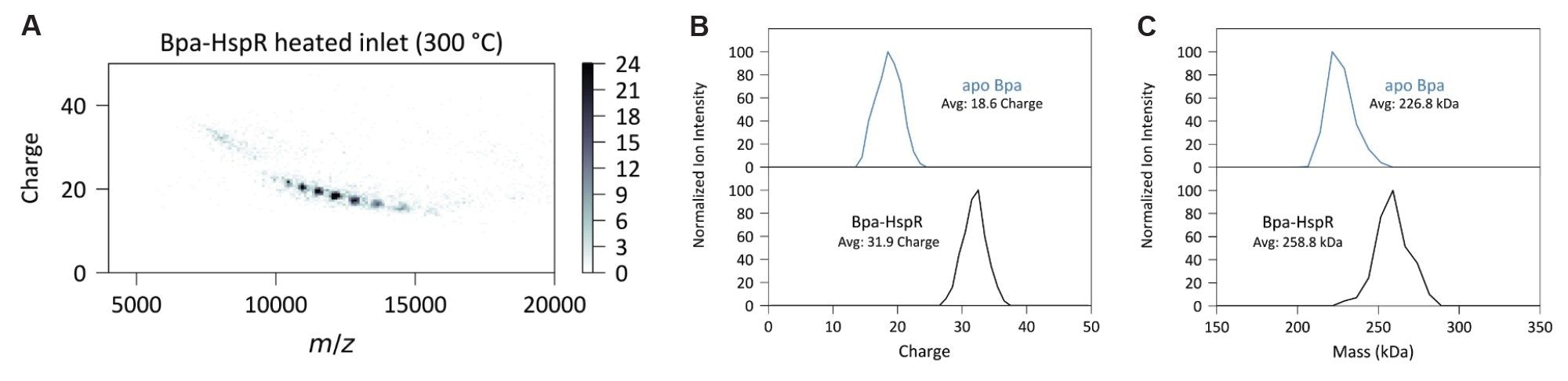
The heated inlet induces Bpa-HspR complex dissociation (Figure 4A). Experiments with the heated inlet showed charge-reduced apo Bpa dodecamers, consistent with asymmetric charge partitioning of the Bpa:HspR complex due to HspR ejection. The Bpa-HspR complex had an average charge of 31.9 which is reduced to 18.6 for apo Bpa upon HspR dissociation (Figure 4B).
Utilizing a heated inlet during ion transfer demonstrates the ability to obtain highly accurate mass measurements of heterogeneous high-mass ions. The heated inlet also allows improved desolvation and facilitates ion activation of the 20S CP and proteasomal regulatory particles. Incorporating inlet heating into the CDMS instrument enhances structural resolution and expands our ability to probe the architecture and mechanisms of biologically relevant noncovalent protein assemblies.
Conclusion
The availability of a commercial CDMS instrument is critical for advancing research into large protein complexes. Traditional MS techniques often struggle with the accurate determination of mass for large biomolecular assemblies due to their inherent heterogeneity, high molecular weight, and low signal response. A commercial CDMS system allows researchers to directly measure both the m/z ratio and charge of individual ions, enabling the precise mass characterization of complex entities such as viruses, antibodies, and protein assemblies. This capability accelerates discovery in structural biology, virology, and biopharmaceutical research by providing reliable, reproducible data that was previously only accessible to specialized labs. The transition from custom-built prototypes to commercially available CDMS instruments democratizes powerful analytical methods, driving innovation and deeper mechanistic insights into biological systems.
Xevo CDMS enables the characterization of large protein complexes that previously could not be analyzed using traditional MS. The inclusion of the heated inlet within the CDMS system allows for the observation of previously unavailable insights such as heat-induced dissociation and the resulting charge reduction. Inlet heating effectively induces complex dissociation, as demonstrated by charge-reduced Bpa dodecamers and asymmetric charge partitioning. For the native 20S CP, however, it maintained its expected intact mass until heating above 250 °C triggered charge loss and subunit dissociation, where consecutive losses of the α-subunits were observed. This highlights Xevo CDMS as a powerful tool for probing stability and conformational changes in large protein complexes.
References
- Hogan, J. A.; Jarrold, M. F. (2018). JASMS, DOI: 10.1021/jasms.3c00177
- Bolten et.al. (2016) Structure, DOI: 10.1021/j.str.2016.10.008
- Hu. et.al. (2018) J. Biol. Chem, DOI: 10.1074/jbc.RA117.001471
Featured Products
720009071, October 2025