Spectral Analysis of Broad-Spectrum Sunscreens Using the Alliance™ iS HPLC System with Photodiode Array (PDA) Detector
Catharine E. Layton, Paul D Rainville, Amy Woodsmall
Waters Corporation, United States
Published on April 29, 2025
Abstract
In this application note, the Alliance iS HPLC System with Photodiode Array (PDA) Detector is shown to separate and identify compounds, determine spectral purity, and visualize UV absorptive regions for chemical UV filters used in sunscreen lotion formulations.
Benefits
- The Alliance iS HPLC System with PDA Detector consistently shows low carryover and high repeatability for peak retention time and area
- Empower™ Software, when paired with the Alliance iS HPLC System with PDA Detector, can determine peak purity in chromatographic separations
- Chemical UV filters in complex matrices are identified using Empower Software's PDA Library
- Contour and three-dimensional plots provide a visualization of UVC, UVB, UVA, and High-Energy Visible (HEV) blue light broad-spectrum absorbance for sunscreen compounds
Introduction
Ultraviolet (UV) electromagnetic radiation is responsible for a variety of chemical reactions, including photochemical smog, bleaching of paints, and decay of plastics. Conjugated bonds in organic molecules absorb UV radiation to cause damage and oxidative stress to lipids and proteins. This stress results in sunburn, hyperpigmentation, photoaging of the skin, wrinkles, age spots, broken capillaries, and deadly skin cancer. There are three types of solar UV radiation: UVC (100–280 nm), UVB (280–315 nm), and UVA (315–400 nm). The ozone layer absorbs 100% of UVC, 90% of UVB, and a minimal amount of UVA radiation. The depletion of the ozone layer has resulted in increased concern for overall ground-reaching UV exposure.1 Additionally, High-Energy Visible (HEV) blue light next to the UVA region (400–450 nm) has also been shown to cause DNA and cellular damage.2 This radiation is emitted from digital devices, light-emitting diode (LED) light bulbs, and florescent light bulbs. Studies suggest that regular use of broad-spectrum sun protection factor (SPF) sunscreens, i.e., those that absorb both UVA and UVB radiation, can reduce the risk of skin damage induced by both UV and HEV sources of radiation.3
The SPF rating is the minimal dose of a sunscreen formulation that produces perceptible skin erythema using a solar simulated light source.4,5 UV filters in sunscreens provide a physical or chemical protection barrier. Physical filters work by a similar mechanism as clothing. They include titanium dioxide or zinc oxide and sit on top of the skin to act as a reflective or light scattering barrier. Chemical sunscreens reduce damage from UV radiation by the transfer of energy into a chemical reaction. Compound structure typically exhibits an aromatic functional group, conjugated to a carbonyl group. The compound absorbs high-energy radiation to form an excited state. As the molecule returns to the ground state, it releases the absorbed energy into the skin as heat. Chemical sunscreens can protect against UVB and/or UVA radiation. UVB filters include, aminobenzoates, cinnamates, salicylates, octocrylene, ensulizole, and camphor derivatives. UVA filters include benzophenones, anthranilates, avobenzones, and ecamsules.6,7
Both physical and chemical sunscreens, formulated as lotions, oils, sprays, creams, gels, pastes, and sticks must meet Food and Drug Administration (FDA) over-the-counter (OTC) drug product allowances for concentration and compatibility because of manufacturer package claims to prevent sunburn and skin cancer. Lately, sunscreens have been placed under increased scrutiny. For example, chemical ingredients used in the US for many years, trolamine salicylate and aminobenzoic acid (PABA), are now recognized as unsafe or ineffective, and environmental research has shown evidence that oxybenzone and octinoxate cause harm to aquatic environments. Internationally accepted sunscreens, such as bemotrizinol, used for more than 20 years in Japan, South Korea, Europe, and Australia, are still under evaluation in the US for safety, efficacy, and environmental impact. A greater understanding of UV radiation filters formulated as sunscreens across the globe is pertinent.
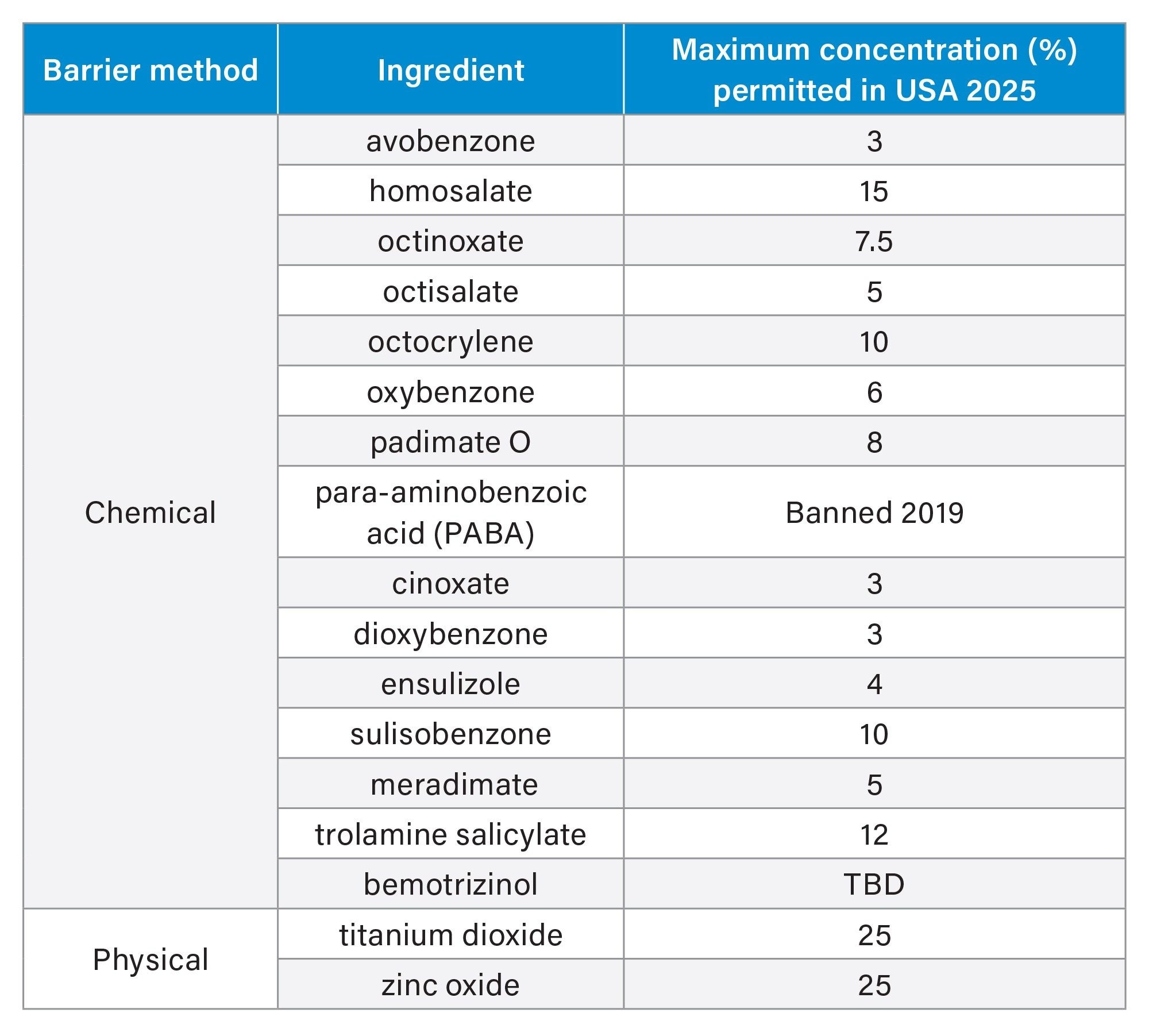
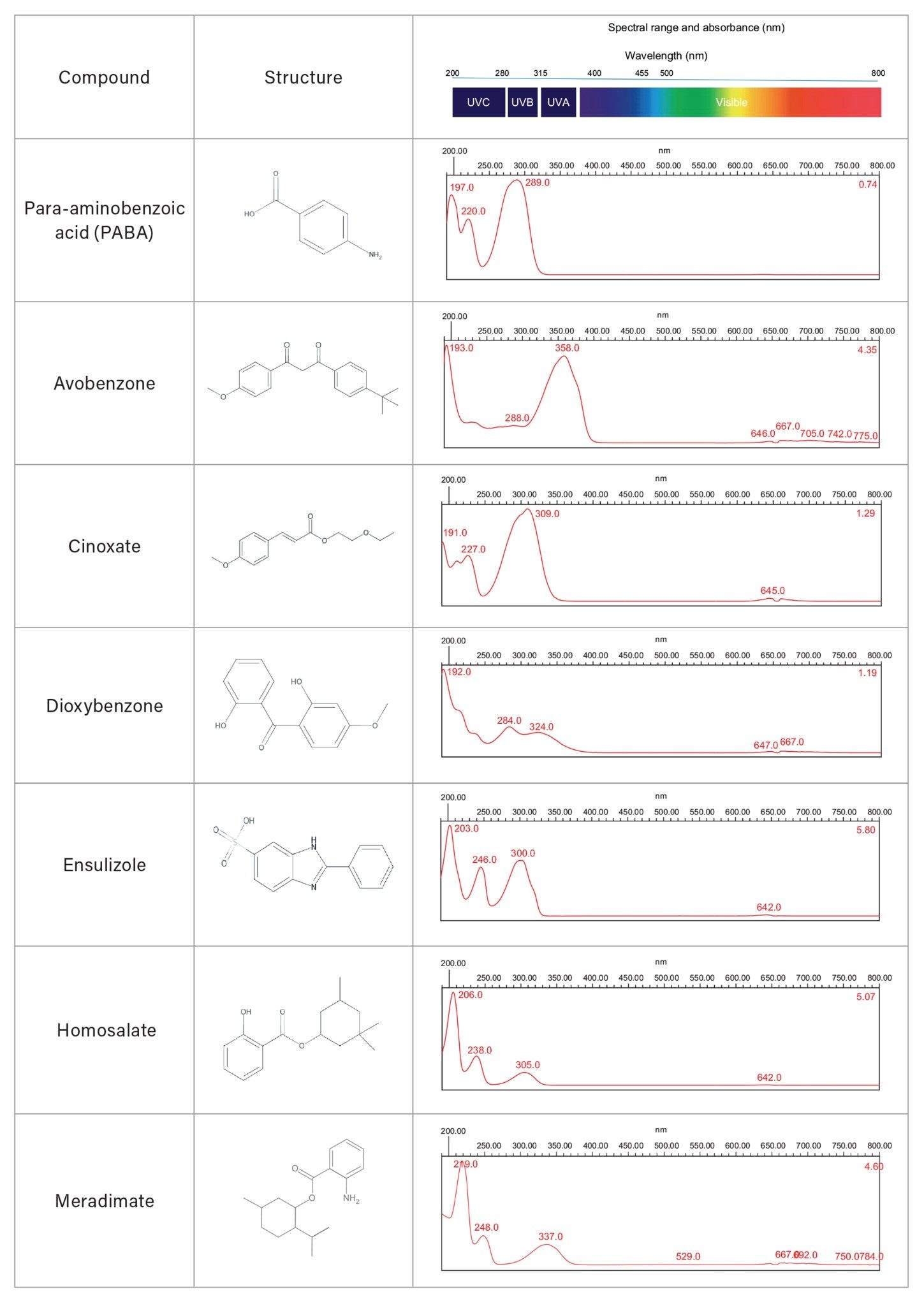
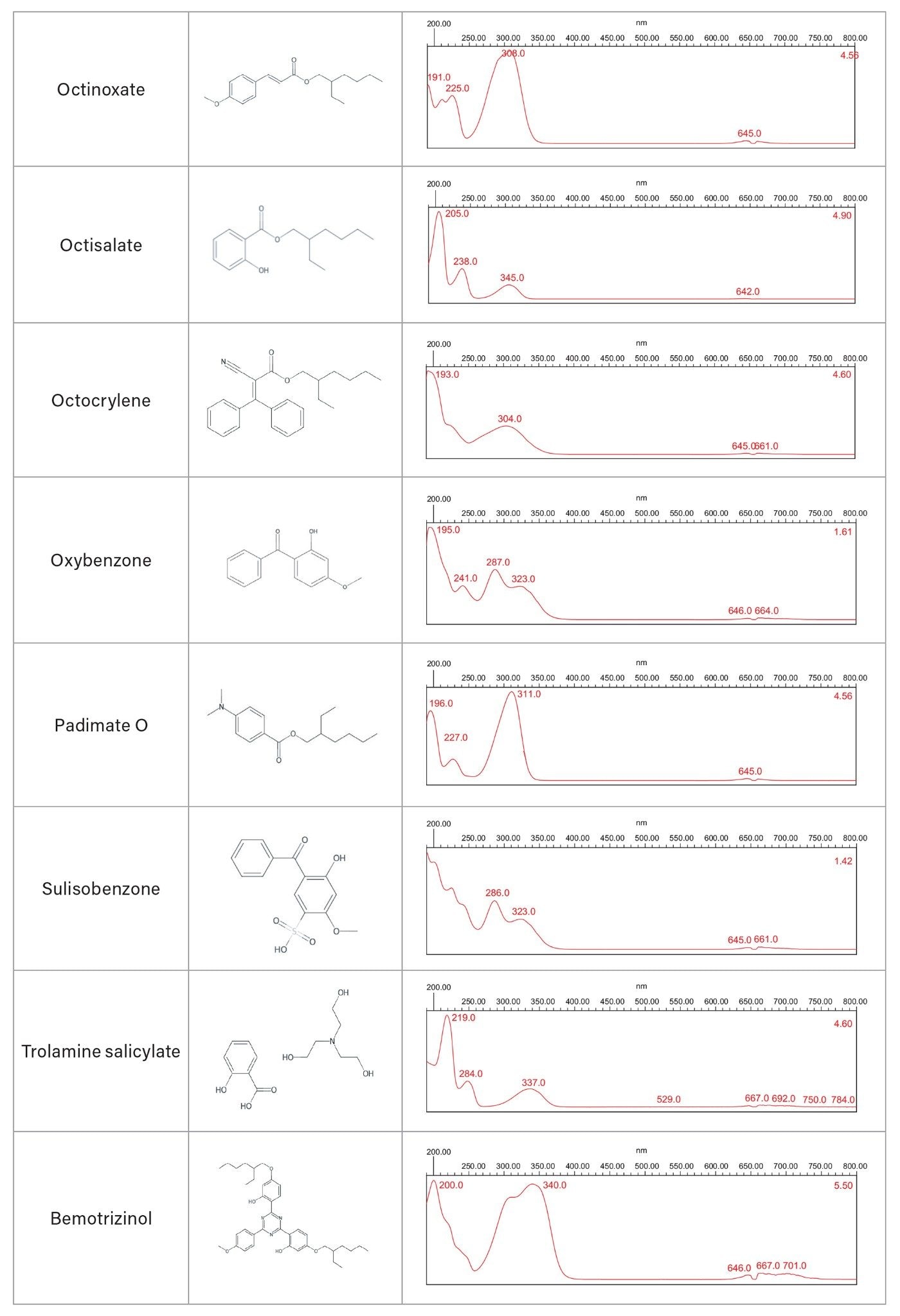
Chemical sunscreens can be quantified in liquid formulations using liquid chromatography (LC), while physical sunscreens, often inert minerals, require other techniques such as Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). In this application note, the Alliance iS HPLC System with Photodiode Array (PDA) Detector was used to determine identity and chromatographic purity, and visualize the spectral absorbance range for 15 chemical UV filters currently under evaluation for OTC sunscreen formulation approval in the US (Table 1).
Experimental
LC Conditions
|
LC system: |
Alliance iS HPLC System with Photodiode Array (PDA) Detector, software version 1.4.0. |
|
Column: |
XBridge™ Premier BEH™ C18 2.5 µm, 4.6 x 150 mm, p/n: 186009849 |
|
Column temperature: |
40 °C |
|
Sample temperature: |
20 °C |
|
Injection volume: |
35 µL |
|
Flow rate: |
2.000 mL/min |
|
Mobile phase A: |
Water/0.1% formic acid |
|
Mobile phase B: |
Acetonitrile/0.1% formic acid |
|
Gradient for formulation analysis: |
Hold for one minute at 70% mobile phase B, linear gradient to 95% mobile phase B over four minutes, then re-equilibrate to starting conditions |
|
Run time(s): |
Seven minutes for reference standard mixture, 15 minutes for lotion samples |
|
Diluent: |
Ethanol sample dilution, methanol prior to injection |
|
Sample filter: |
0.2 µm PTFE CE Acrodisk Minispike Filter, (p/n: WAT200556) |
|
Needle wash: |
50/50 methanol/water |
|
2D wavelength: |
254 nm |
|
3D wavelengths: |
190–800 nm |
|
Resolution: |
1 nm |
|
Data rate: |
10 Hz |
|
Extracted channel: |
254 nm |
|
CDS: |
Empower 3.8.0 |
Sample Preparation
Fifteen chemical UV filter reference standards were solubilized in methanol to equal a concentration of approximately 4.0 mg/mL. Individual PDA spectra were collected from reference standard injections, and a PDA Library created in Empower Software. A reference standard mixture containing avobenzone (3%), homosalate (13%), octisalate (5%), octocrylene (10%), and oxybenzone (6%) was prepared in methanol. Three OTC sunscreen lotions were weighed and dissolved in ethanol by sonication to equal a final concentration of approximately 5 mg/mL (w/v). The supernatant was diluted 1:10 in methanol, and 1 mL filtered with a 0.2-µm filter prior to injection. Six replicate injections of each sunscreen lotion solution were performed. System repeatability, carryover, peak purity, and PDA Library identification were determined from the lotion injections.
Results and Discussion
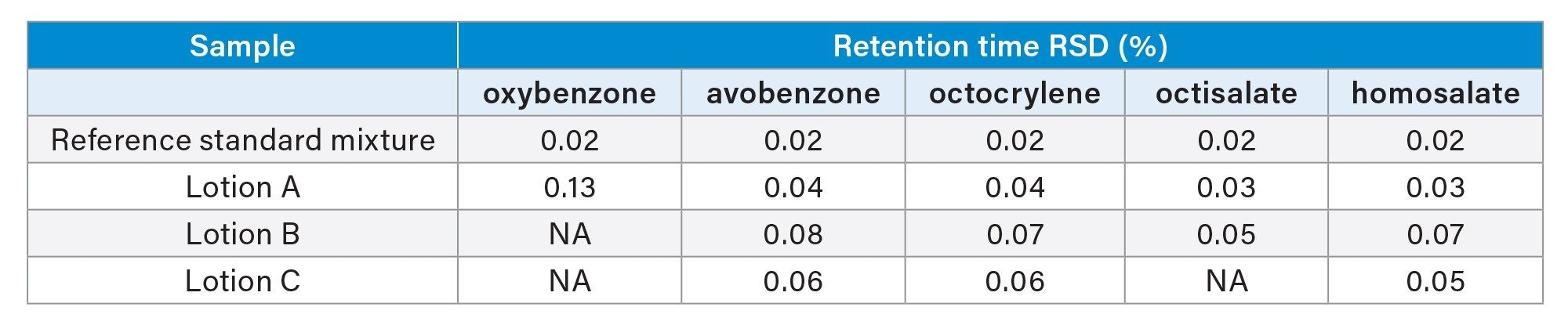
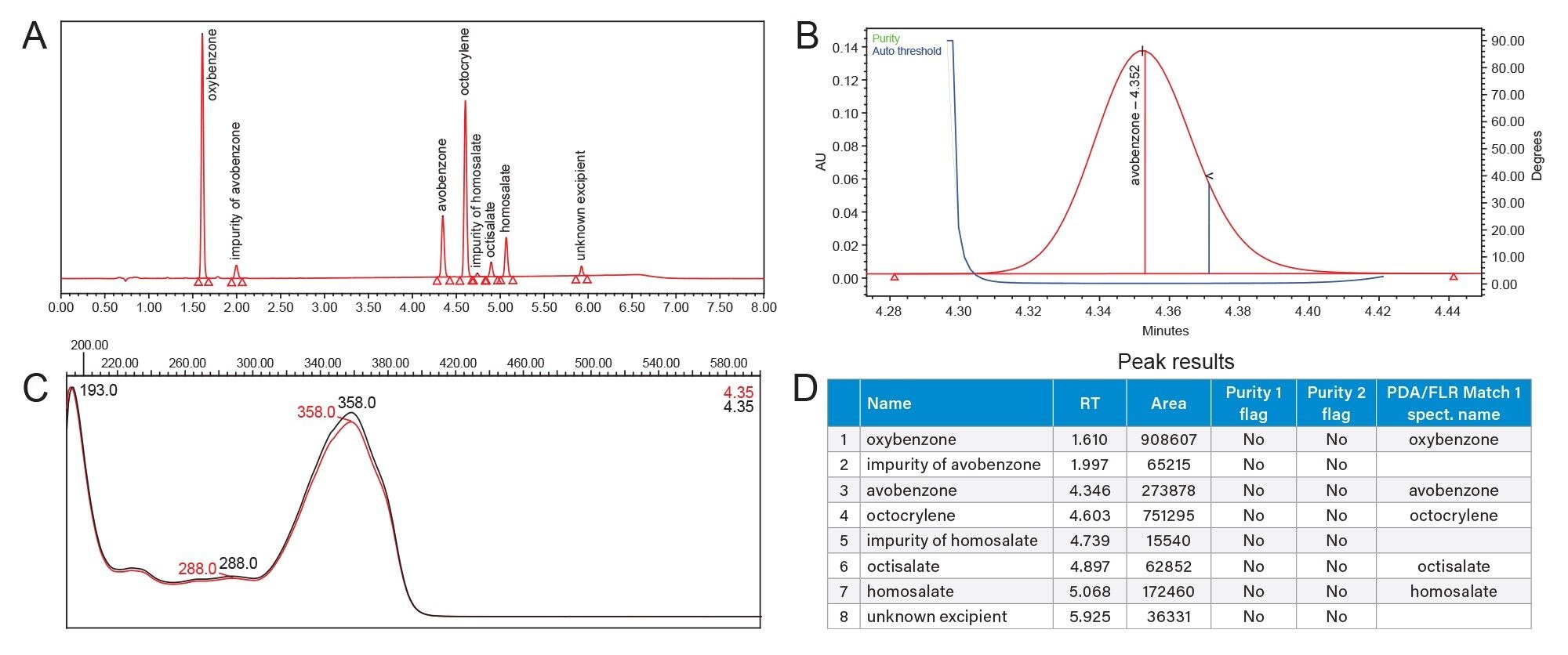
The HPLC method separation baseline resolved the five UV filters in the reference standard mixture (oxybenzone, avobenzone, octocrylene, octisalate, and homosalate) and two related impurities in 5.5 minutes (Figure 1). Retention time repeatability for six replicate injections of the mixture was ≤0.02 RSD (Figure 2, Table 2) and ≤0.19 RSD for peak area (data not shown). These results were well below the typical method validation acceptance of ≤2.0% RSD. Injection carryover was not observed for the reference standard mixture.
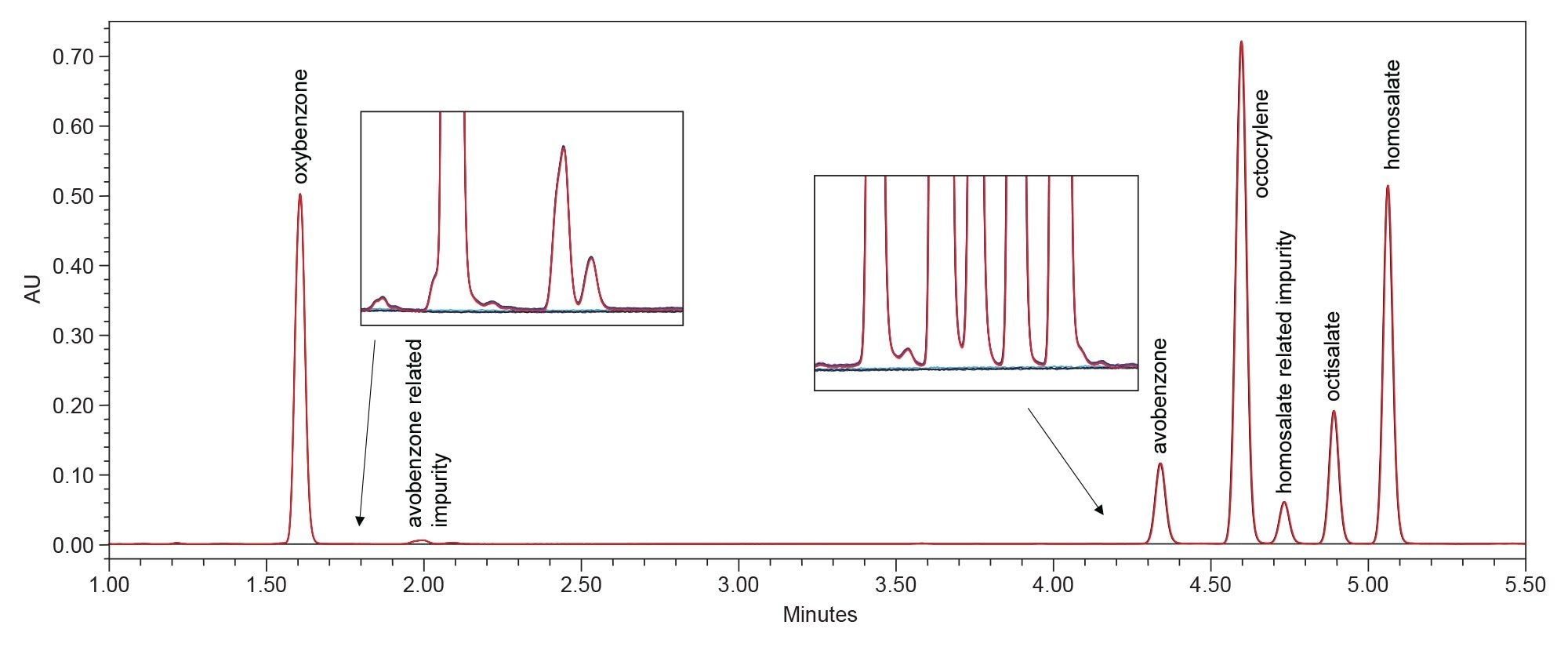
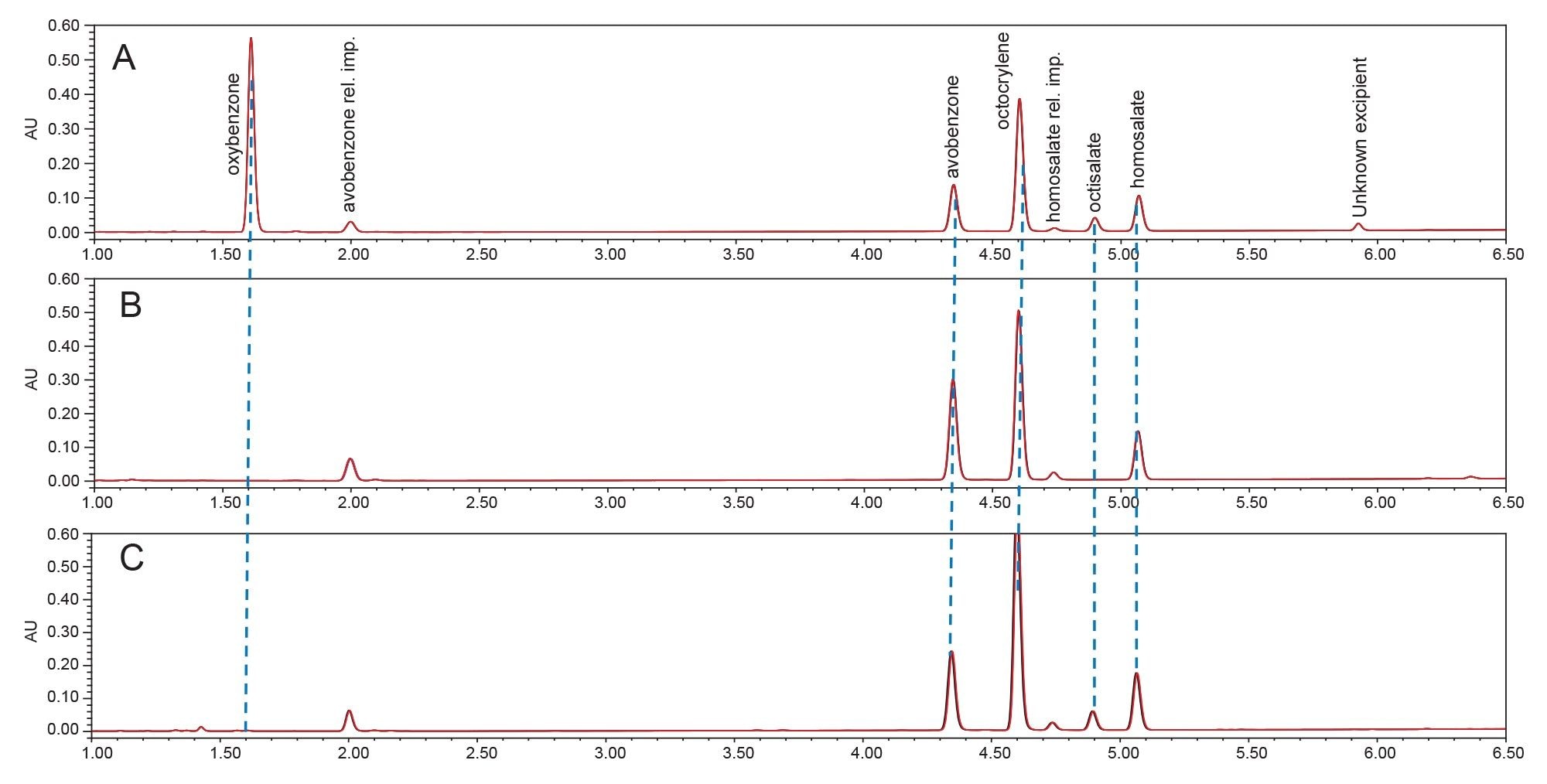
The spectra of 15 individual UV filter reference standards revealed lambda max within the UVC, UVB, and/or UVA electromagnetic range. None of the sunscreen compounds showed high absorbance in the HEV blue light spectral range (Table 3). Eluting sunscreen lotion peaks were determined to be spectrally homogenous by the Empower 3 Software. This was accomplished automatically by comparing spectra gathered across the eluting peaks with the noise threshold generated by the solvent (Figure 3). UV filter peaks were instantly recognized using the PDA Library, which utilized both the retention time and spectral profile of the reference standards for identification. Sunscreen Lotion A peaks were labeled by the PDA Library as: avobenzone (UVA/UVB), homosalate (UVA), octisalate (UVB), octocrylene (UVB), and oxybenzone (UVA/UVB). Sunscreen Lotion B contained four UV filters: avobenzone, homosalate, octisalate, and octocrylene, and Sunscreen Lotion C contained avobenzone, homosalate, and octocrylene.
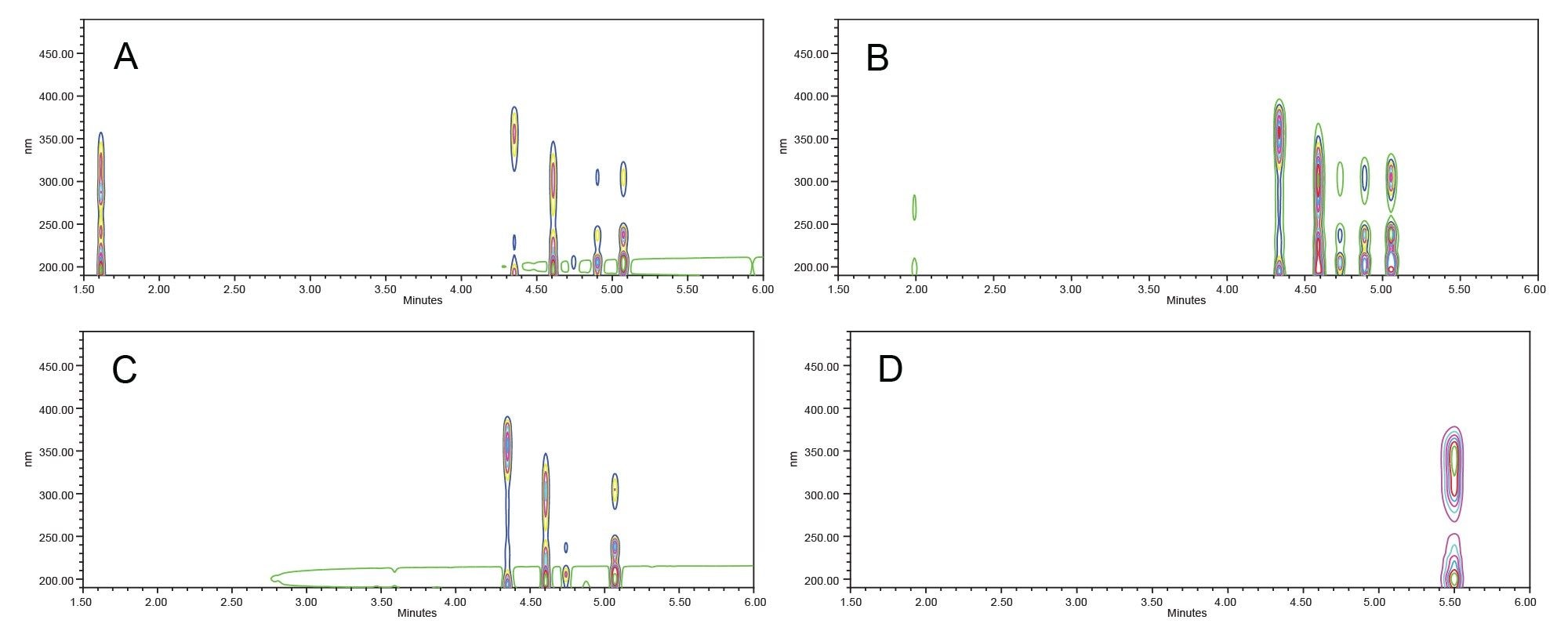
Contour (Figure 4) and three-dimensional (Figure 5) plots were used to comprehensively visualize the broad-spectrum UV filter absorbance. Sunscreen lotions, comprised of multiple UV filters, showed absorbance across both the UVB and UVA range. Bemotrizinol, a sunscreen used in formulations internationally, filtered UV radiation in both the UVA and UVB range, but showed less UV coverage around 275 nm.
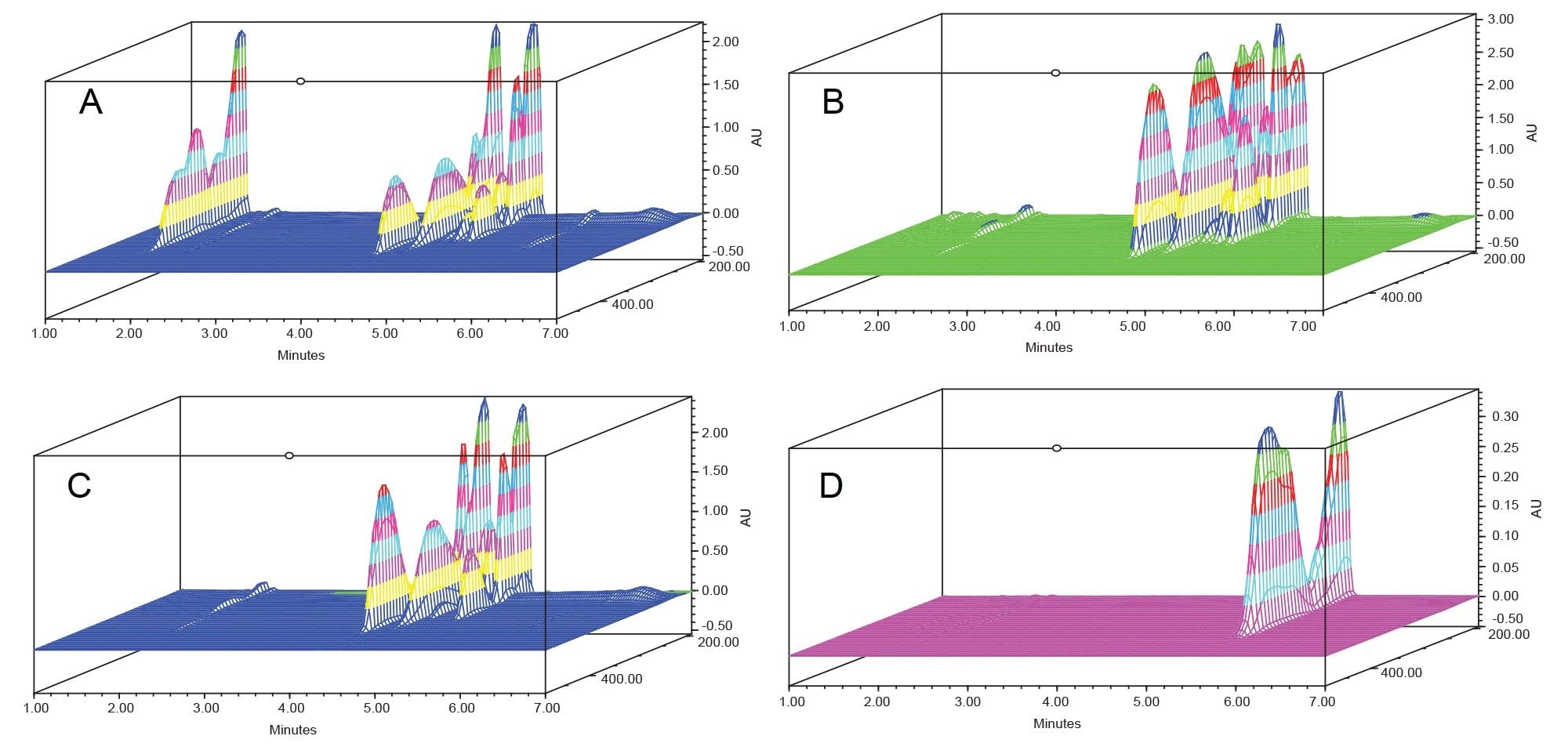
Conclusion
The Alliance iS HPLC System with PDA Detector provided key peak purity and spectral information. Repeatability of the Alliance iS HPLC System was higher than method validation requirements, and no injection needle carryover was observed. All 15 compounds showed absorbance of UVA and/or UVB radiation, while absorbance within the HEV blue light was minimal. For the three sunscreen lotions tested, spectral purity was confirmed for all peaks eluting in the chromatographic separation, and UV filters were automatically identified using Empower Software's PDA library. Spectral absorbance plots, contour plots, and three-dimensional plots provided easy to interpret, full-spectrum visualization of UV and HEV radiation coverage for both individual UV filters and formulated sunscreen lotions.
References
- de Gruijl FR, van der Leun JC. Environment and Health: 3. Ozone Depletion and Ultraviolet Radiation. CMAJ. Oct 3;163(7):851–5. 2000.
- Coats JG, Maktabi B, Abou-Dahech MS, Baki G. Blue Light Protection, Part I-Effects of Blue Light on the Skin. Journal of Cosmetic Dermatology. Mar;20(3):714–717. 2021.
- Conant, L., Beck, K.M., Liao, W., A Rapid and Cost-Effective Device for Testing Minimal Erythema Dose. Dermatology Theory (Heidelb). 8, 483–489. 2018.
- Gabros, S., Zito, P.M., Sunscreens and Photoprotection. StatPearls (Internet). StatPearls Publishing. 2019.
- Osterwalder, U., Herzog, B., Chemistry and Properties of Organic and Inorganic UV Filters. 2008.
- In Lim, H.W., Draelos, Z.D. (Eds.), Clinical Guide to Sunscreens and Photoprotection. Informa Healthcare, New, York, pp. 11–38. 2009.
- Osterwalder U, Herzog B. Sun Protection Factors: World-wide Confusion. Br J Dermatology Nov;161 Suppl 3:13–24. 2009.
- Chemical Structures, https://www.chemspider.com/Chemical-Structure.953.html, (accessed 14:03, Dec 12, 2024).
720008733, April 2025