Applying Analytical Method Greenness Scoring to the USP Monograph of Naproxen; Improving the Sustainability of Validated Methods by Modernizing to Newer Technology
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Sustainability improvements are hard to implement for validated methods, particularly if the method calls for specific solvents to be used in order to achieve the desired results. Unlike new method development activities, validated methods, like USP monograph methods, are locked in and cannot be changed without significant work to re-validate. There is one path towards higher sustainability for these methods: modernization. By implementing USP General Chapter <621> principles for modernization, a validated method can be scaled to newer instrumentation, leading to reductions in both run time and solvent usage. In addition to the practical benefits of increasing throughput and reducing operational costs, this has the secondary benefit of improving the sustainability of the method.
This application note examines the modernization of the UPS monograph of naproxen previously reported.1 In addition to revisiting the previous work, Analytical Method Greenness Scores (AMGS) will be calculated for the assay on each system used. This will provide a measure of sustainability to prove that modernizing older monograph methods not only provides the benefits of lower run times and less solvent used, but also greatly improves the sustainability of running one such assay.
Benefits
- Four-fold reduction of analytical run time and solvent usage by modernizing to UHPLC instrumentation and columns
- Up to 13-fold decrease in solvent usage and eight-fold decrease in run time by modernizing to UPLC systems
- More sustainable methods without the need to revalidate the method
- Higher throughput with greatly reduced operational cost
Introduction
The importance of sustainability has grown, to the point where efforts to make scientific processes “greener” have been implemented. Unfortunately, for certain analytical workflows, creating a brand new “green” method to analyze a sample may not always be viable or possible. Using alternative bio-based solvents like ethanol may not always provide the required selectivity and resolution for a liquid chromatography method. Additionally, in some cases, an analytical method has already been validated and implemented, further reducing the possibility of making changes. Re-validation of an existing method is challenging and may only be worth the additional time and money when a method is not performing properly.
The laboratories using validated methods, typically for quality control, are often stuck with these methods, which can often employ older instrumentation and column technology. One example of this is for USP monograph methods which have been in place for many years, and were originally published when HPLC systems and columns packed with 5 µm particles were the standard for analysis. Now however, both instrumentation and column technology has improved. These improvements may not be needed for all methods, but their adoption brings a number of benefits. First and foremost, the newer technologies, like UHPLC and UPLC systems coupled with an appropriate column often bring lower run times and less solvent usage. This directly translates to lower operational costs and higher throughput. Additionally, this reduction in solvent usage and instrument run time also means that the method has become more sustainable. The energy needed to run the system is reduced overall with short run times, and less solvent used means less waste. Modernization of older methods designed on HPLC instruments is one way to make these validated methods more sustainable, reducing their environmental impact. To demonstrate how modernization affects sustainability, the USP monograph of naproxen tablets is examined. This work was previously reported, and showed significant improvements to time and cost of analysis over the lifetime of a column.1 By using this example and applying Analytical Method Greenness Scores (AMGS), the improvements in sustainability can be added to the benefits of modernization.
AMGS values were first introduced in 2019 by Hicks et.al.2 This metric considers not only the instrument being used, but also the samples being analyzed, analytical run times, and solvents used. A free calculator is available on the ACS Green Chemistry Institute Pharmaceutical Roundtable Website and was used extensively for this application note.3 The previously reported modernization results for naproxen sodium tablet were used to calculated AMGS values across the three LC platforms used.
Results and Discussion
Previously, the USP monograph assay method for naproxen sodium tablets was modernized from HPLC technology to newer technology, specifically smaller particle size columns on UHPLC and UPLC systems.1 The USP monograph conditions were first replicated on an HPLC system to ensure acceptable results. The mobile phases, column configuration, flow rate, detection, and other parameters were all applied.4 Figure 1 shows the resulting chromatograms obtained using three different systems for the assay.
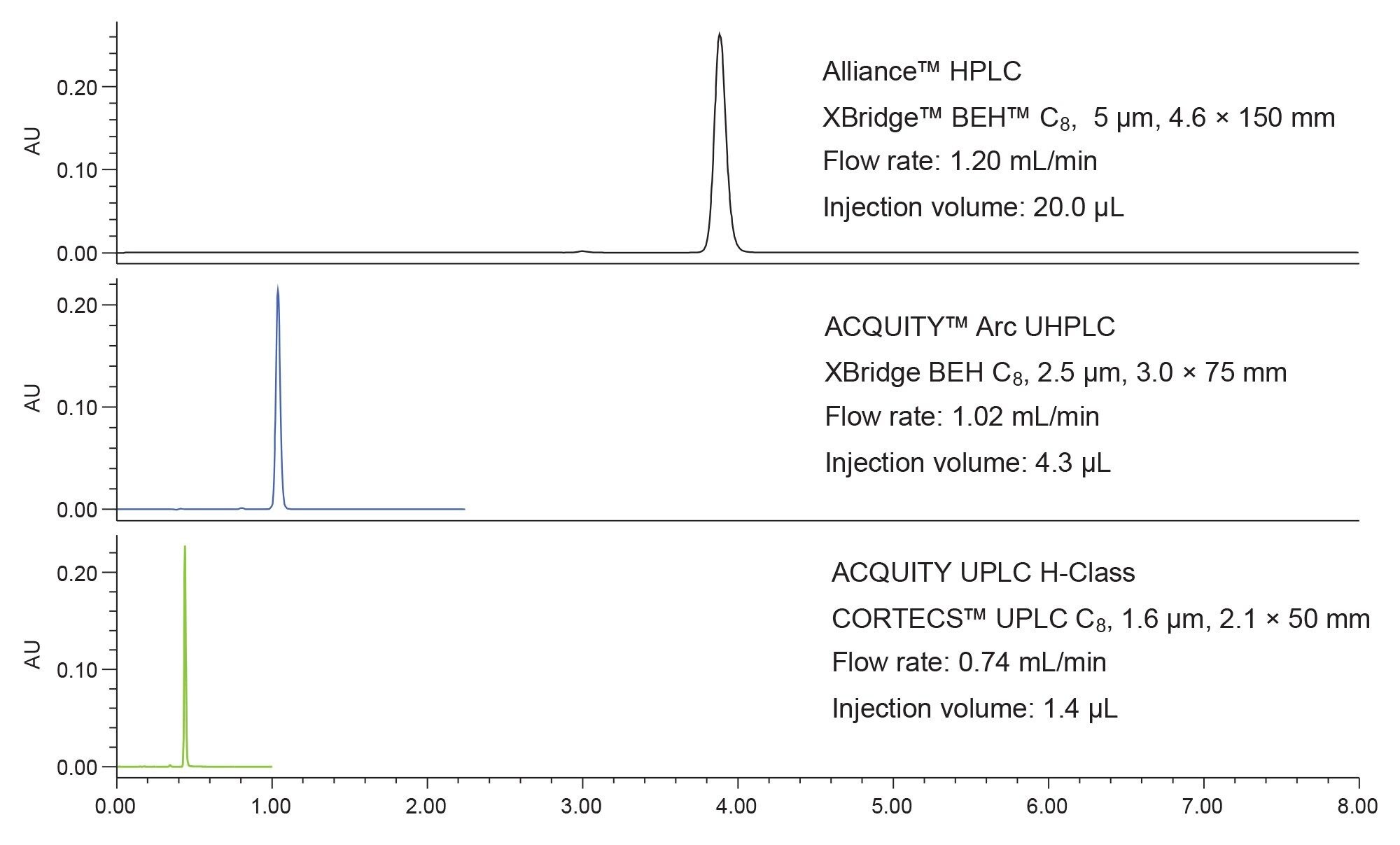
Across the three platforms, all system suitability requirements were met, including reproducibility for the standard, and calculated concentration of a formulated sample. Next a lifetime study was performed to determine the cost and time savings of this assay over the operational life of the sub-2 µm column. After 10,000 injections of formulated sample and standard the column was still performing well, and the lifetime study was halted. Calculating the number of days needed to achieve 10,000 injections as well as the cost of creating the mobile phase needed for 10,000 injections led to the results shown in Table 1.
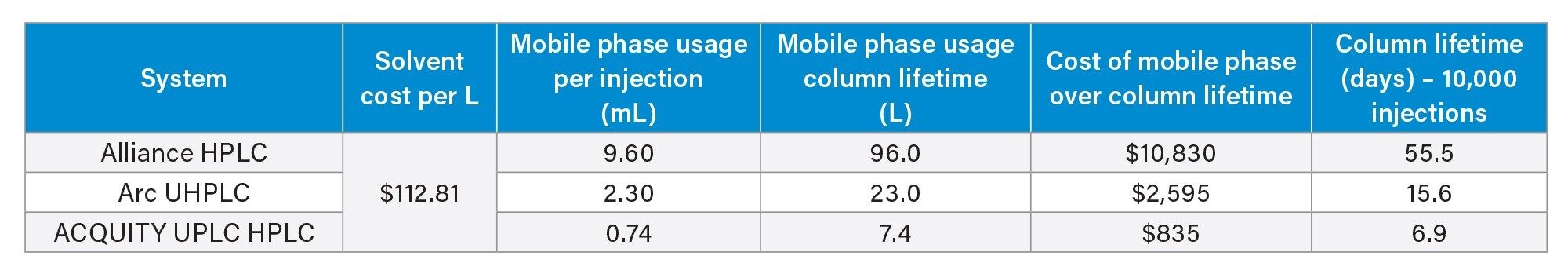
The modernization process could have yielded a savings of almost 10,000 dollars over the lifetime of a single column in terms of solvent cost. This does not factor in the cost of waste removal for the solvent consumed. Additionally, these calculations show that the UPLC method requires only seven days for 10,000 injections, while the HPLC method needs 55 days. The cost and time savings of modernization are very pronounced for this assay. However, sustainability was never examined.
For sustainability calculations, only one “batch” of testing will be examined. For one batch of sample, a total of five injections of the system suitability standard is needed, and at least three replicates of the formulated sample would be needed. These replicates ensure proper data collection for the calculations required. When applying the AMGS metric, only that number of injections will be used.
When using the calculator, the user must first select what technique is used. For this example, HPLC will be selected for both the HPLC system and the UHPLC system, which is not present in the list. Next values for the number of analytes of interest, 1, and the number of injections needed for an analysis, 8, are input. The next section covers the instrument conditions, such as flow rate and run time. For the HPLC system, these values are 1.2 mL/min and eight minutes. Isocratic conditions of 45:54:1 acetonitrile:water:glacial acetic acid are next needed. However, the calculator does not have acetic acid in the drop down menu, so the input values were 45:55 acetonitrile:water.
The next section examines samples, including sample diluent and stock solutions needed. For this example, the prepared volumes of both samples and standards were calculated based on the injection volume of the system and the number of replicates needed. For instance, using the HPLC system and 20 µL injections, the standard volume needed is 100 µL, while the sample volume needed is 60 µL. While not practical from a laboratory standpoint, by performing the calculations using these values, not only will solvent use and run time be considered, but also sample consumption across the three platforms. The above-described steps were performed for each system used in modernization resulting in the AMGS values shown in Table 2.
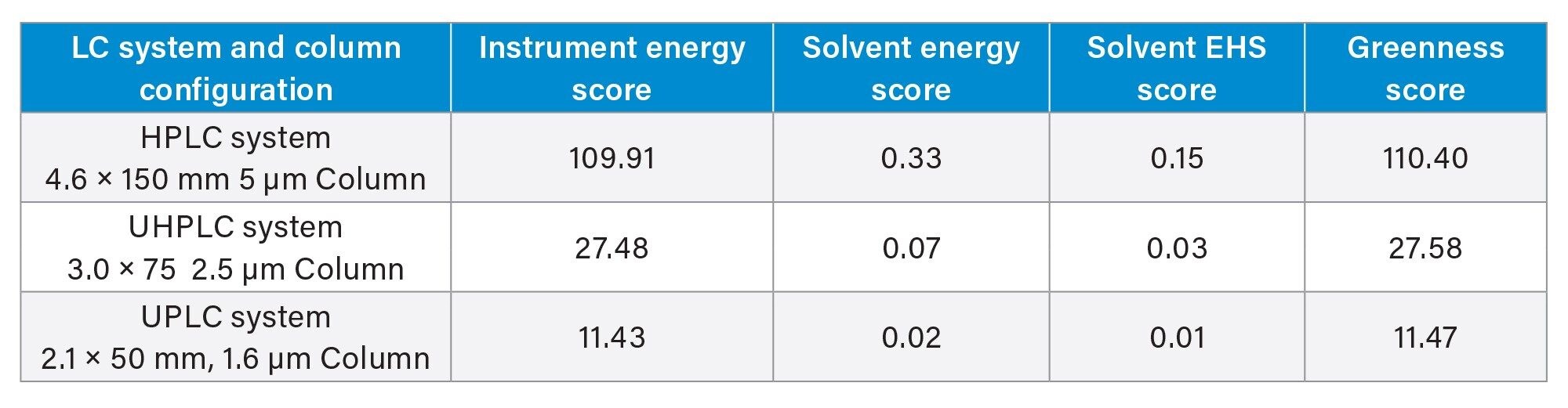
The biggest contributor to the AMGS values for a given technique is the instrument energy score, which factors in both the type of system, run times of the samples, and number of injections required. As expected, the HPLC method which has the longest run times has the highest instrument energy score. The HPLC method also has the highest solvent energy and solvent EHS scores. This results in a Greenness Score of 110, while both the UHPLC and UPLC methods result in Greenness Scores under 30.
Modernization is not always a simple process and requires a lot of forethought into whether it is the right thing for a laboratory. However, for those labs that do decide to modernize their older methods, not only can significant improvements in run time and solvent usage be realized, but the sustainability of the lab can also be improved.
Conclusion
Sustainability efforts are becoming a driving force in many LC workflows. Method development activities are beginning to factor in “Greenness”, especially when multiple methods meet the scientific criteria for the method. However, for validated methods, which are already in use, sustainability efforts are harder to realize. One path to sustainability for these types of methods is to ensure that modern LC system and column technology is being used. By standardizing on UHPLC or UPLC systems with small particle size columns, significant improvements to the “Greenness” of a method can be achieved.
To demonstrate this, a previously modernized USP assay monograph for naproxen sodium tablets was examined from the sustainability perspective. Using the Analytical Method Greenness Score (AMGS) metric across three LC platforms it was determined that the use of the UPLC method with sub-2 µm particle columns is the most sustainable. This is driven primarily by reductions in run time and flow rate, i.e. solvent usage, which also translates into higher throughput and lower operational costs. If a laboratory is not able to modernize to UPLC systems, moving to UHPLC systems with appropriate column configurations can still greatly improve the “Greenness” of the method.
References
- Berthelette K, Nguyen J, Turner J, Savage D. Reductions in Cost and Time by Modernizing a USP Monograph Method from HPLC to UHPLC and UPLC Instrumentation and Columns. Waters Application Note. 720007427, November 2021.
- Hicks M, Farrell W, Aurigemma C, Lehmann L, Weisel L, Nadeau K, Lee H, Moraff C, Wong M, Huang Y, Ferguson P. Making the Move Towards Modernized Greener Separations: Introduction of the Analytical Method Greenness Score (AMGS) Calculator. Green Chem. 21 (2019) 1816.
- ACS Green Chemistry Institute Pharmaceutical Roundtable Website. https://acsgcipr.org/amgs/ Accessed 14-Feb-2024.
- USP Monograph for Naproxen Sodium Tablets. https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/iras/naproxen-sodium-tablets.pdf Accessed 23-Feb-2024.
- Calculation of Cost Per L of Mobile Phase.
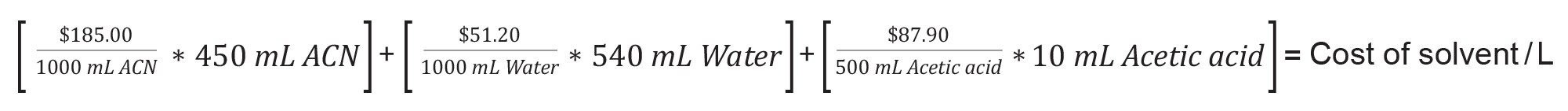
Featured Products
720008366, May 2024