For research use only. Not for use in diagnostic procedures.
The analysis of Azole antifungals in serum was described in this application note.
Use of UPLC-MS/MS enables separation of itraconazole and voriconazole from their metabolites, and the selectivity provided by mass selective detection provides a reliable means of analysis of antifungal compounds in serum for clinical research purposes.
Here described is a method for the analysis of azole antifungals in serum. This method may be used for emerging indications, and for understanding pharmacokinetic and pharmacodynamic properties in clinical research.1,2 Although microbiological test methods are in use to measure azole antifungals, enhanced activity of the itraconazole metabolite – hydroxyitraconazole – can overestimate concentrations.1,2 Similarly, the use of two or more drugs in combination can impair the utility of microbiological test methods.1 Furthermore, measurement of hydroxyitraconazole is of unknown utility and remains the subject of research.3
The method described utilizes deproteination of serum samples with a deuterated internal standard mixture in methanol. Separation was achieved within three minutes using an ACQUITY UPLC BEH C18 Column on an ACQUITY UPLC I-Class System followed by detection on a Xevo TQD Mass Spectrometer (Figure 1).

Standards were sourced for fluconazole, itraconazole, posaconazole, and voriconazole (Sigma-Aldrich, Dorset, UK); hydroxyitraconazole and voriconazole-N-oxide (Toronto Research Chemicals). Stable labeled internal standards 2H4-fluconazole, 2H5-hydroxyitraconazole, 2H5-itraconazole, 2H4-posaconazole, 2H3-voriconazole, and 2H3-voriconazole-N-oxide were sourced from Toronto Research Chemicals.
Calibrators were prepared in pooled serum purchased from Golden West Biologicals (California, USA). The calibration range was 0.5–100 μg/mL for fluconazole and 0.05–10 μg/mL for all other compounds. QC materials were also prepared in pooled serum at 1.5, 20, and 80 μg/mL fluconazole and 0.15, 2, and 8 μg/mL for all other compounds.
To 50 μL of sample, 950 μL of internal standard in methanol containing 0.1% formic acid (1 μg/mL 2H4-fluconazole, 200 ng/mL for all other internal standards) was added, vortex-mixed, and centrifuged for two minutes at 16,100 g. Supernatant (50 μL) was diluted with 150 μL water to prepare the final extract for analysis.
|
System: |
ACQUITY UPLC I-Class (FTN) |
|
Needle: |
30 μL |
|
Column: |
ACQUITY UPLC BEH C18, 130Å, 1.7 μm, 2.1 mm x 30 mm |
|
Mobile phase A: |
Water + 2 mM ammonium acetate + 0.1% formic acid |
|
Mobile phase B: |
Methanol + 2 mM ammonium acetate + 0.1% formic acid |
|
Needle wash solvent: |
80% aqueous methanol |
|
Purge solvent: |
Mobile phase A |
|
Seal wash: |
20% aqueous methanol |
|
Column temp.: |
50 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.80 mL/min |
|
Time(min) |
% Mobile phase A |
% Mobile phase B |
Curve |
|---|---|---|---|
|
Initial |
75 |
25 |
Initial |
|
2.1 |
3 |
97 |
7 |
|
2.5 |
75 |
25 |
11 |
|
Run time: |
3.0 min (3.7 min injection-to-injection) |
|
System: |
Xevo TQD |
|
Resolution: |
MS1 (0.7 FWHM) MS2 (0.7 FWHM) |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) (see Table 1 for details) |
|
Polarity: |
ESI+ ionization |
|
Capillary: |
0.8 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Inter-scan delay: |
0.02 s |
|
Inter-channel delay: |
0.01 s |
MassLynx v4.1 with TargetLynx Application Manager
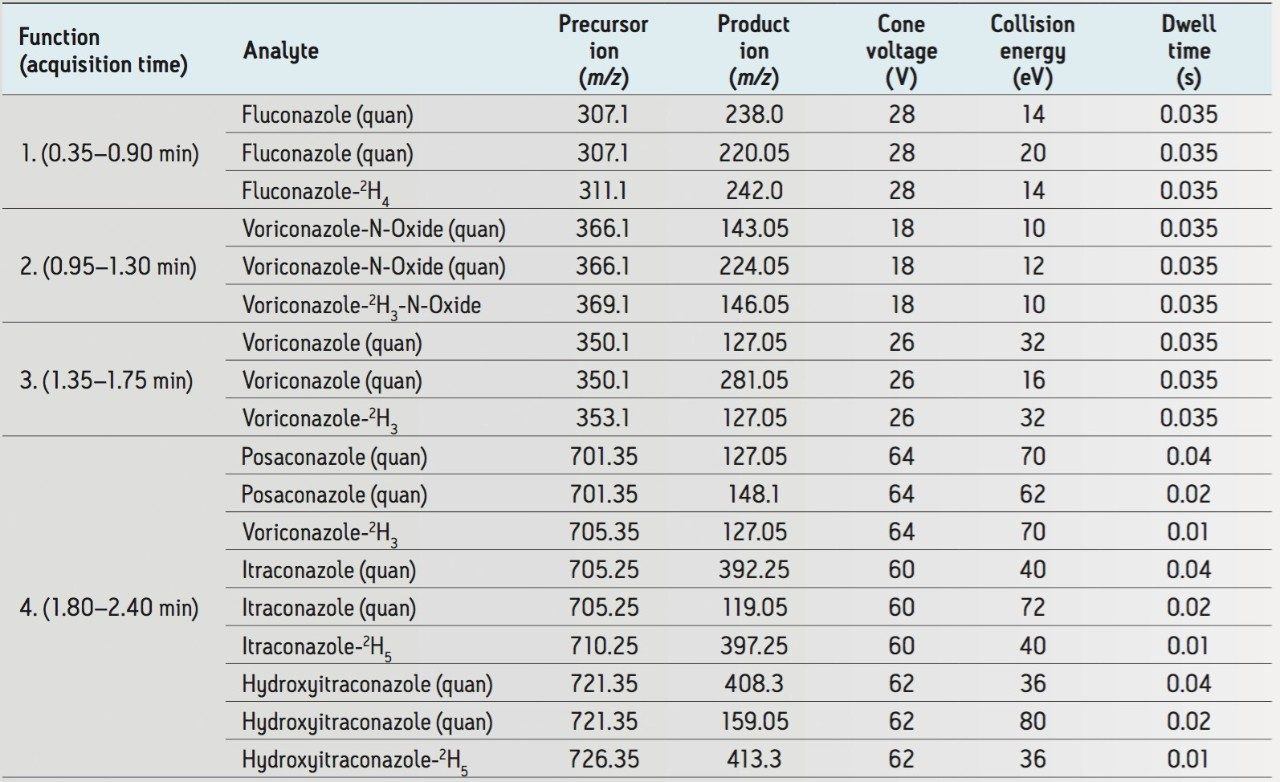
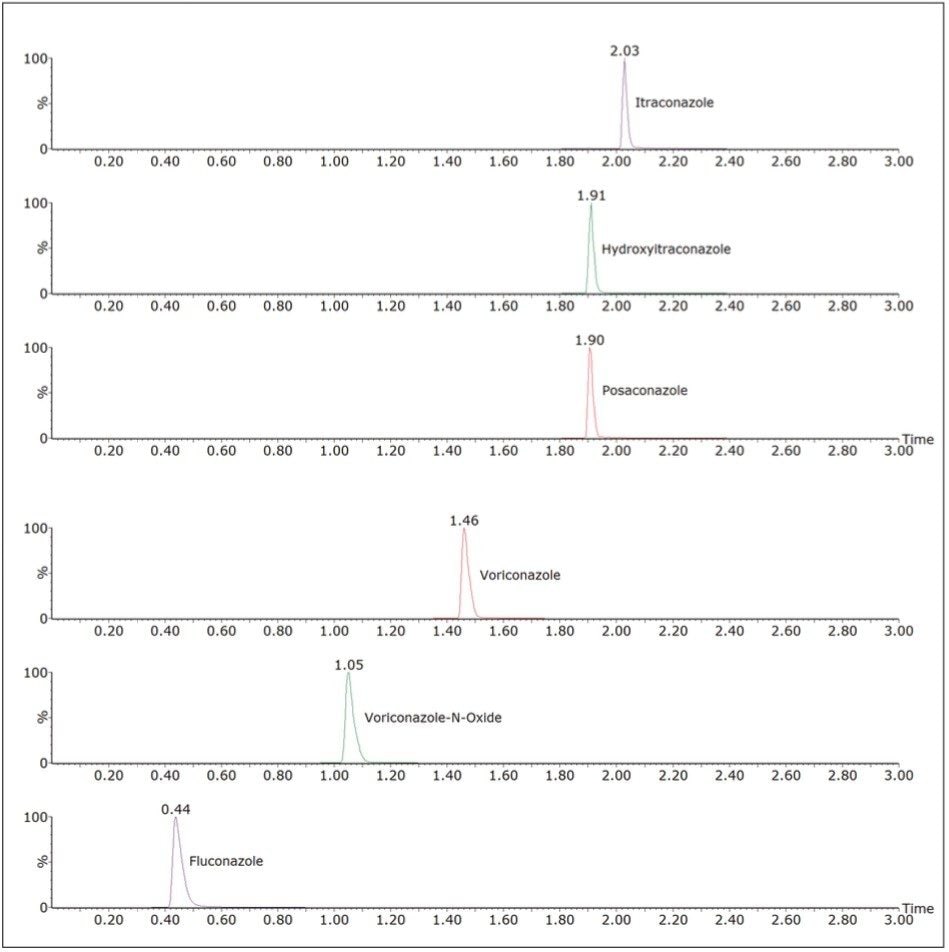
Under these chromatographic conditions, all compounds are separated chromatographically, with the exception of hydroxyitraconazole and posaconazole, which are separated by mass. Figure 2 shows a mid-range calibrator (50 μg/mL fluconazole, 5 μg/mL all other compounds). No carryover was observed for any compounds.
Analytical sensitivity investigations demonstrate that the method would allow precise quantification (<20% RSD) at 0.375 μg/mL for fluconazole, 0.05 μg/mL for hydroxyitraconazole and posaconazole, 0.0375 μg/mL for voriconazole-N-oxide, and 0.025 μg/mL for voriconazole.
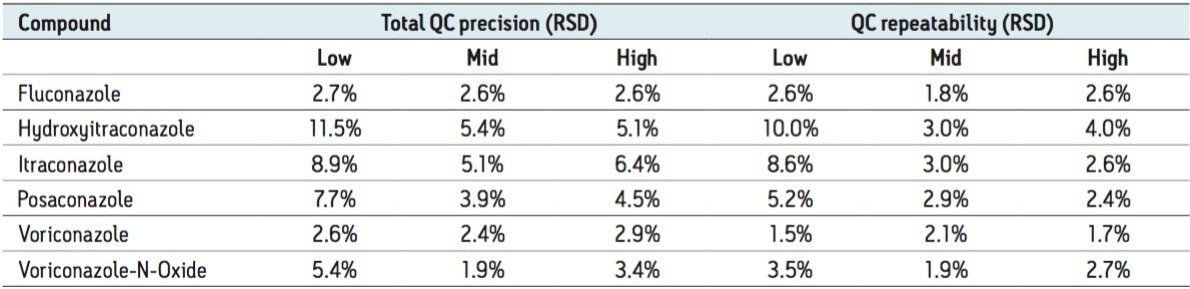
Total precision was determined by extracting and quantifying five replicates of three concentrations of QC material over five separate days (n=25). Repeatability was assessed by analyzing five replicates at each QC level. Table 2 presents results of these experiments, where total precision and repeatability at the low (1.5 μg/mL fluconazole, 0.15 μg/mL other compounds), medium (20 μg/mL fluconazole, 2 μg/mL other compounds), and high (80 μg/mL fluconazole, 8 μg/mL other compounds) concentrations were ≤11.5% RSD.
The method was shown to be linear over the range of 0.457–117 μg/mL for fluconazole, 0.0457–11.7 μg/mL for hydroxyitraconazole and voriconazole-N oxide, 0.381–11.7 μg/mL for itraconazole and posaconazole, and 0.0381–13.0 μg/mL for voriconazole when different ratios of high and low concentration pools were combined and analyzed.
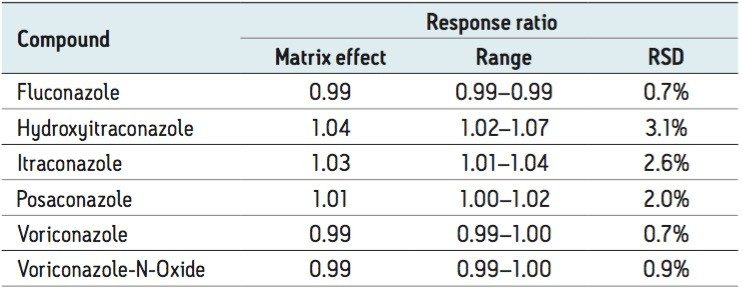
Matrix effects were evaluated as the peak area of extracted post-spiked serum samples (n=6) taken as a percentage of extraction solvent samples spiked to equivalent concentrations. The internal standard was shown to compensate for significant signal enhancement observed for hydroxyitraconazole, itraconazole, and posaconazole, as shown in Table 3 for the response ratio matrix effect.
Potential interference from endogenous compounds (albumin, bilirubin, cholesterol, triglycerides, and uric acid), Intralipid (20% emulsion), and potentially co-administered compounds (cyclosporine, everolimus, mycophenolic acid, sirolimus, and tacrolimus) were assessed by determining the recovery of each compound from a serum pool of known concentration when spiked with the potential interference (n=3). Recoveries ranged from 85.0 107.2% for all compounds.
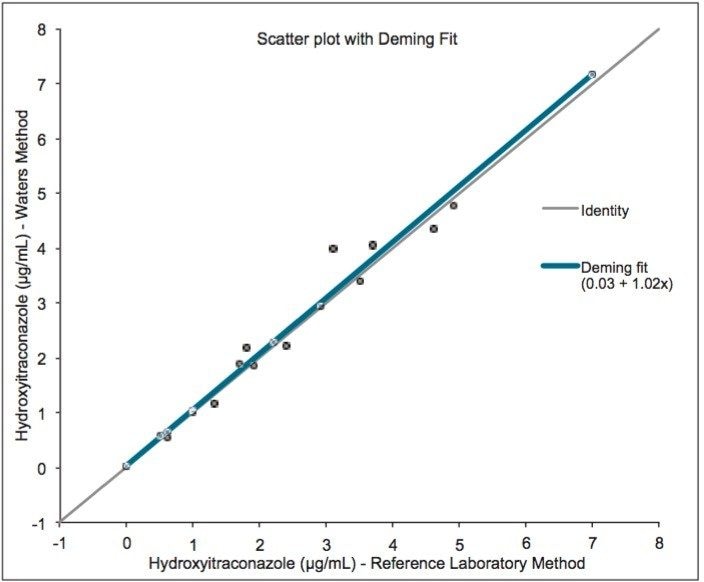
20 serum samples were purchased from a US national reference laboratory with assigned values for hydroxyitraconazole. Good agreement was demonstrated between the Waters UPLC-MS/MS method and the method used by the reference laboratory.
Use of UPLC-MS/MS enables separation of itraconazole and voriconazole from their metabolites, and the selectivity provided by mass selective detection provides a reliable means of analysis of antifungal compounds in serum for clinical research purposes.
This method provides sufficient analytical sensitivity to analyze low levels of fluconazole (0.5 μg/mL), hydroxyitraconazole, itraconazole, posaconazole, voriconazole, and voriconazole N-oxide (all 0.05 μg/mL) over a large linear range (200-fold) using only 50 μL of sample. Sample preparation is simple, fast, and inexpensive.
720005662, March 2016