This application note demonstrates, a robust, selective, and sensitive analytical method for the quantification of 1,25 (OH)2 Vitamin D2 and D3 from human serum was developed using the Waters ACQUITY UPLC System and Xevo TQ-S micro Mass Spectrometer. The Xevo TQ-S micro has the ideal combination of sensitivity, reproducibility, and versatility to provide an excellent option for all types of bioanalytical labs.
Vitamin D is an important fat-soluble vitamin, which helps maintain bone health. Vitamin D exists in two primary forms: Vitamin D3 (cholcalciferol) synthesized from 7-dehydrocholesterol when the skin is exposed to UV radiation from sunlight, and Vitamin D2 (ergocalciferol) produced by plants and fungi through solar irradiation of ergosterol. Vitamin D is converted first to 25-hydroxyl (OH) Vitamin D by the liver via the CYP family of enzymes (Figure 1). 25 (OH) 25 (OH) Vitamin D is then hydroxylated into its biologically-active form: 1,25-dihydroxy (OH)2 Vitamin D in the kidneys.
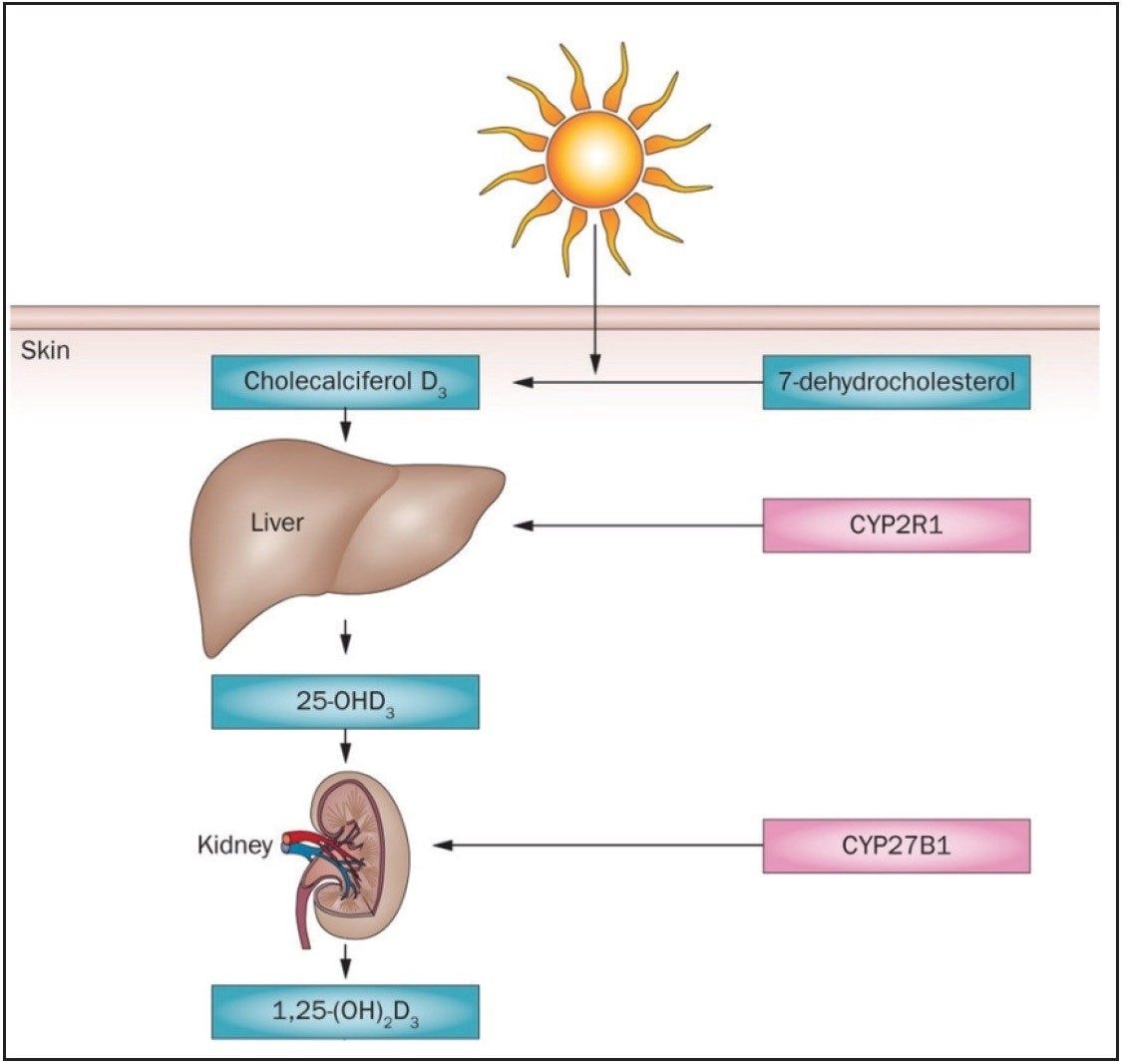
This conversion is tightly controlled through a cascade pathway which involves calcium, phosphorous, parathyroid hormone, and Vitamin D receptors. Regulated by a feedback mechanism process, 1,25 (OH)2 Vitamin D circulates in the pg/mL-level range in serum. Main application areas for the measurement of Vitamin D are in nutrition, pharmacokinetic studies, clinical studies, and quality control for foods and supplements.1
In recent years, LC-MS/MS assays – compared to immunoassays – have gained popularity as the method of choice for quantification of 1,25 (OH)2 Vitamin D. LC-MS/MS assays provide orthogonal selectivity. The identity of every compound is based upon its retention time as well as its unique MRM transition. The addition of immunoaffinity using specific antibodies in sample cleanup adds an extra layer of selectivity to the assay.
LC-MS/MS is accepted by the FDA as the gold standard analytical technique for pharmacokinetic studies of small molecules. The FDA has set specific guidelines to be followed while developing and validating LC-MS/MS assays. These guidelines involve intra-day (within the same day) and inter-day (across multiple days) accuracy and precision studies, linearity, and reproducibility. The method presented here followed the Bioanalytical Method Validation guidelines set out by the FDA. Accuracy, precision, linear range, and reproducibility of the method was evaluated, and the method met the criteria detailed in the FDA guidelines.3
The data presented in this application note was generated using a Waters ACQUITY UPLC System and Xevo TQ-S micro Mass Spectrometer.
Commercially-available immunopurification kits were purchased from ALPCO Diagnostics and used to extract 1,25 (OH)2 Vitamin D from 500 μL of human serum. The eluate from the last step was evaporated to dryness. Samples were derivatized with PTAD (4-phenyl-1,2,4-triazoline-3,5-dione) using 100 μL of 0.75 mg/mL PTAD in acetonitrile, which was added to each tube and allowed to incubate in the dark – at room temperature – for one hour. PTAD was then evaporated using a CryoVac system. The contents of the tubes were reconstituted using 50 μL of 50:50 water–methanol mix. This solution was then transferred to LC MS/MS vials, and 20 μL were injected into a column.
|
Instrument: |
Waters ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18 , 1.7 μm, 2.1 mm x 50 mm (P/N 1860002350) |
|
Column temp.: |
60 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
20 μL |
|
Flow rate: |
0.500 mL |
|
Mobile phase A: |
100% water, 0.1% formic acid, 2 mM methylamine |
|
Mobile phase B: |
100% methanol, 0.1% formic acid, 2 mM methylamine |
|
Gradient: |
Start with 50% A and hold for two minutes. Change to 80% B between 2–4 minutes. Followed by one minute of flushing and one minute of equilibration. |
|
Instrument: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+ |
|
Transitions: |
1,25 (OH)2 Vit D3 – 574.2>314.1 1,25 (OH)2 Vit D2 – 635.3>314.1 1,25 (OH)2 Vit D3 Int std – 580.3>314.1 1,25 (OH)2 Vit D2 Int std – 641.3>314.1 |
|
Capillary voltage: |
2.5 kV |
|
Cone voltage: |
25 V |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas: |
1000 L/Hr |
|
Cone gas: |
25 L/Hr |
MassLynx 4.1
1,25 (OH)2 Vitamin D is an important biomarker tested routinely in clinical and bioanalytical laboratories. The extremely low circulating levels of this molecule, coupled with lack of ionization in electrospray ionization mode, make this a challenging assay. The method described here combines affinity-based sample preparation combined with derivatization to increase ionization as an elegant solution for this analytically-difficult molecule.
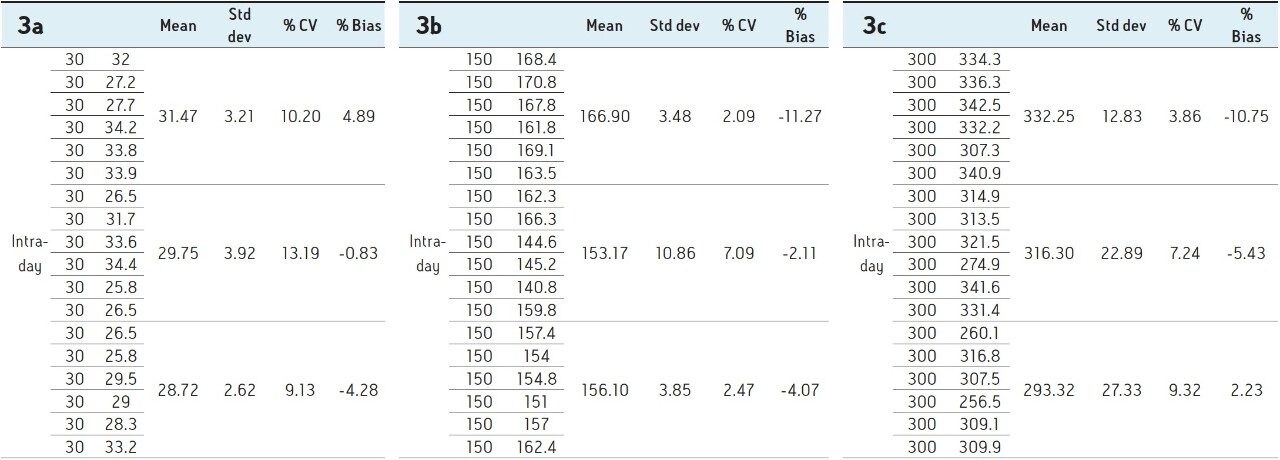
Calibration curve from 5–500 pg/mL is linear and % bias across the range is <15% for both 1,25 (OH)2 Vit D2 and D3 as shown in Figure 3a and 3b.
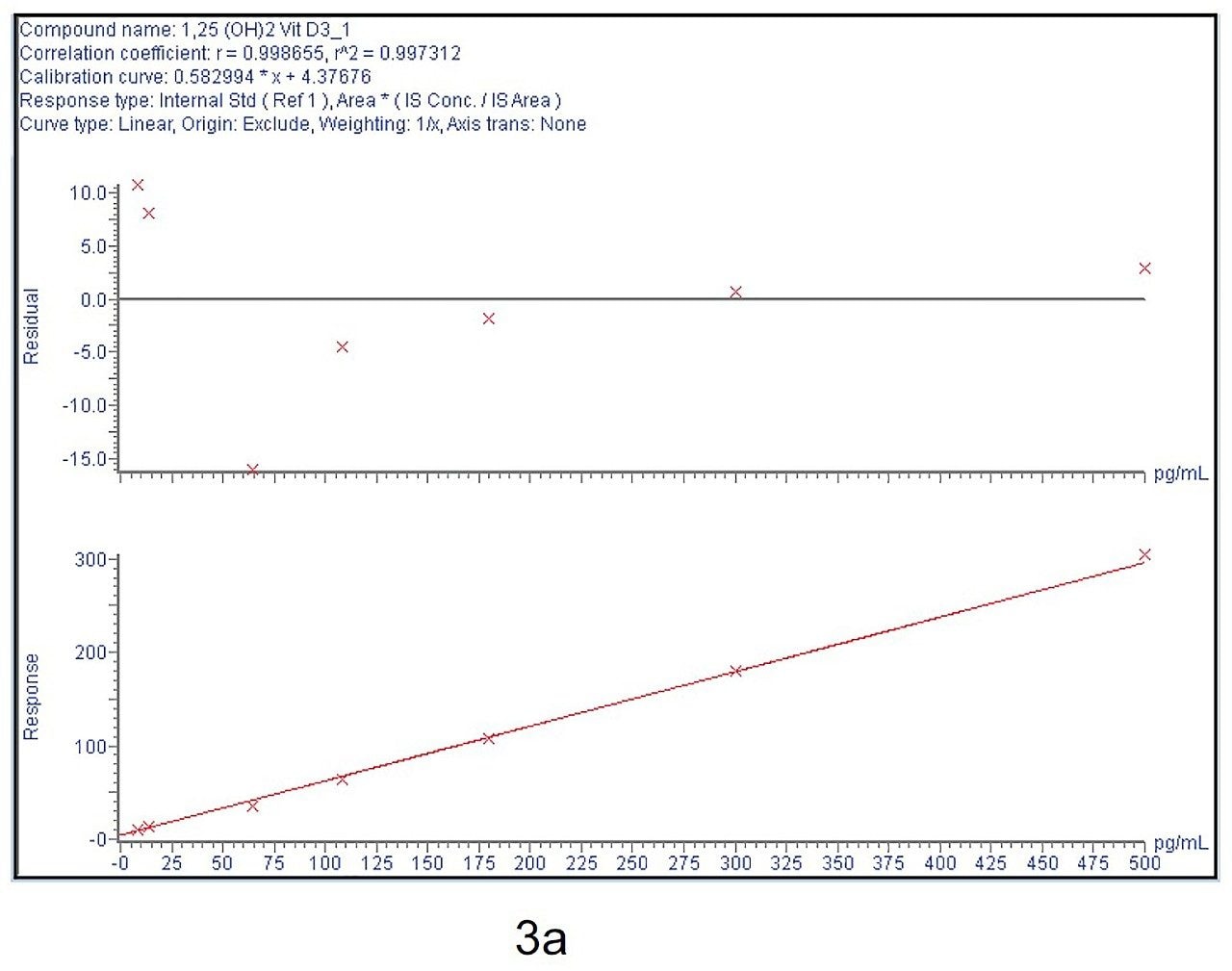
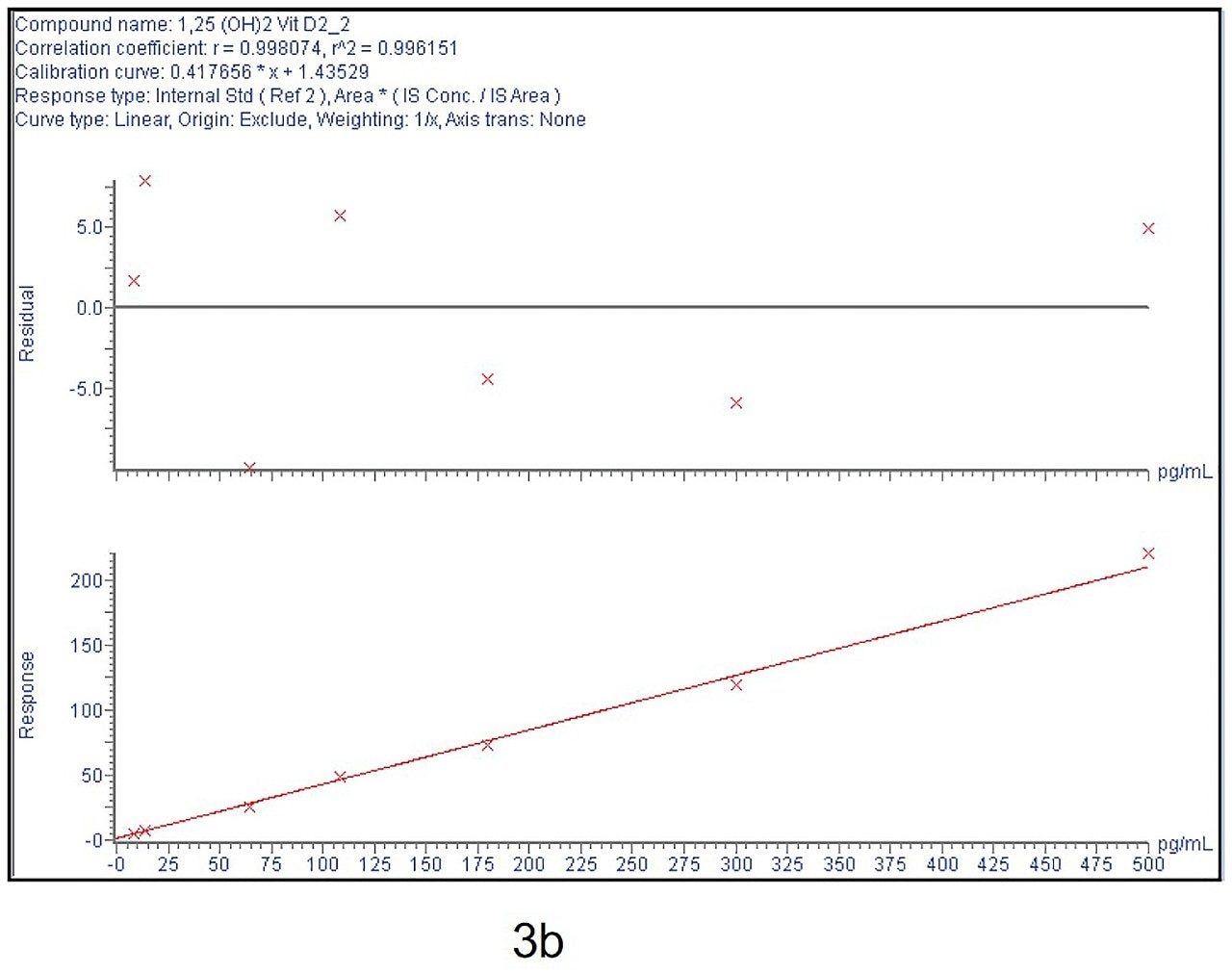
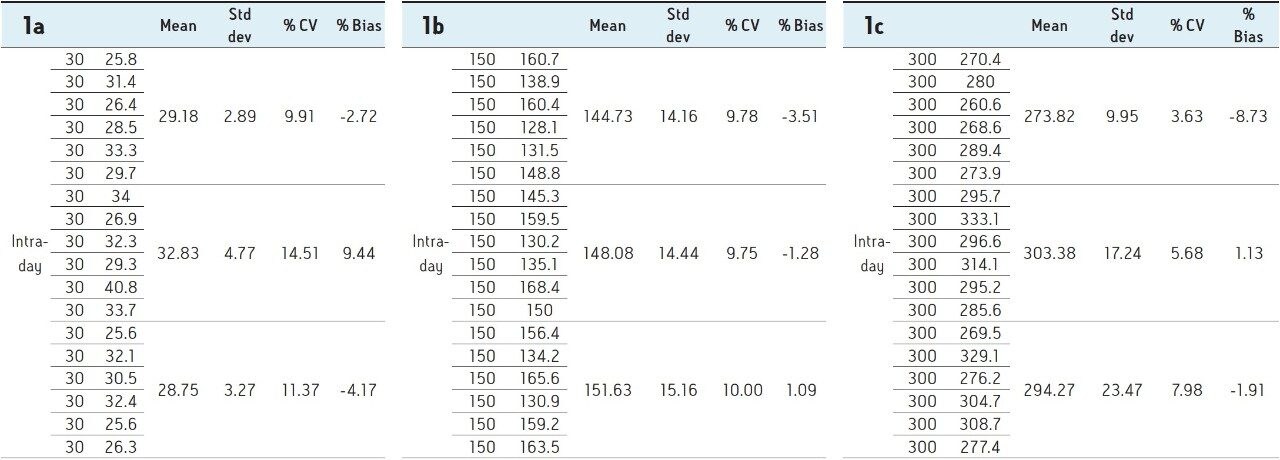
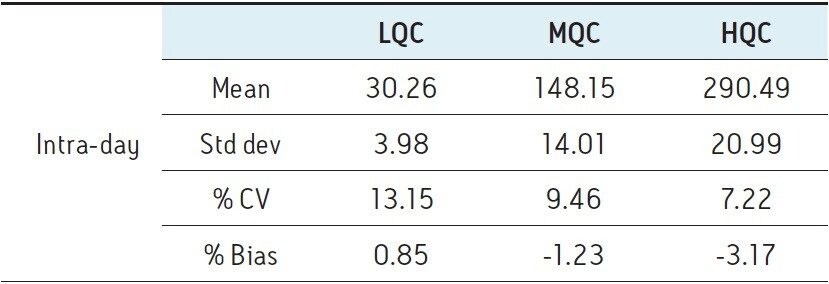
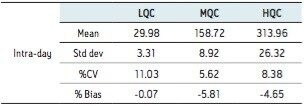
Six replicates at low (30 pg/mL), mid (150 pg/mL), and high (300 pg/mL) QC levels were extracted and injected across three days. The intra-day and inter-day precision and accuracy were <15% at all levels as shown in Tables 1–4.
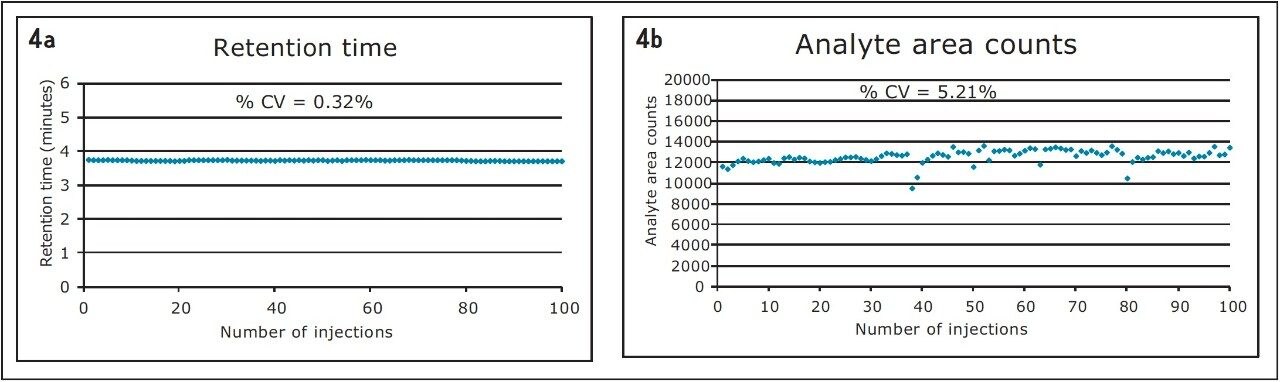
The Waters ACQUITY UPLC and Xevo TQ-S micro displayed robust injection reproducibility as shown below. The % CV for the retention times was 0.32% (Figure 4a) and the % CV for analyte area counts was 5.21% (Figure 4b) – both of which are well within the acceptable criteria.
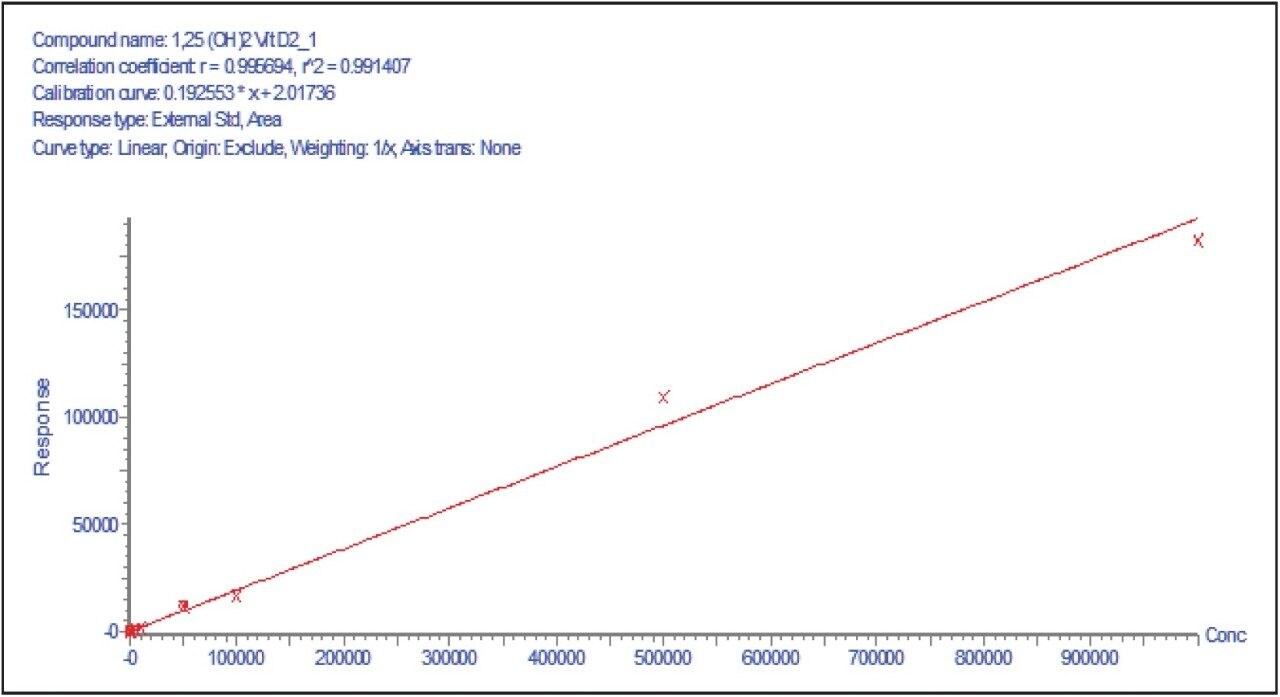
The analytical range for this method is from 5–500 pg/mL and covers the relevant concentrations typically found in serum. 1,25 (OH)2 Vitamin D showed linearity from 50 pg/mL–1 μg/mL using the ACQUITY UPLC and TQ-S micro for the method described here.
A robust, selective, and sensitive analytical method for the quantification of 1,25 (OH)2 Vitamin D2 and D3 from human serum was developed using the Waters ACQUITY UPLC System and Xevo TQ-S micro Mass Spectrometer. A limit of quantification of 5 pg/mL was readily achieved while maintaining excellent linearity. Calibration curves for both 1,25 (OH)2 D2 and D3 were linear over the range of 5–500 pg/mL with r2>0.99. Across three days, the intra- and inter-day CV as well as the % bias were <15% for both 1,25 (OH)2 Vitamin D2 and D3. Injection reproducibility was excellent with % CV <0.32% for retention time and <5.5% for area counts.
Today’s analytical laboratories are becoming more diverse and multi-functional. Lab managers are expected to diversify their analytical platforms within limited lab spaces. The Xevo TQ-S micro has the ideal combination of sensitivity, reproducibility, and versatility to provide an excellent option for all types of bioanalytical labs.
720005679, April 2016