This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates the predictability and robustness of an empirical table created for use in Auto•Blend Plus.
The defined protocol provides two buffer systems, one low pH range and one high pH range, for the specified components of formic acid and ammonia. The two buffer systems can be used for LC-MS analysis if the buffer solutions are prepared exactly as the protocol states. The protocol is reliable in controlling the variability associated with an empirical table and provides the ability to create an empirical table across multiple laboratories.
Two buffer systems were defined, one low pH range and one high pH range, for use in Auto•Blend Plus for LC-MS reversed-phase separations.
Control of mobile phase pH is one element in developing reliable and reproducible chromatographic methods. To provide accurate control of the mobile phase pH during an analysis, Auto•Blend Plus Technology,1 provided in the software, automatically manages the pH and ionic strength components of a defined mobile phase. The ACQUITY UPLC H-Class System includes the function of Auto•Blend Plus in order to generate a specific mobile phase pH on demand from stock solutions. The algorithm that proportions the stock solutions can use an empirical table created within an Auto•Blend Plus method. Based on the algorithm, the Quaternary Solvent Manager (QSM) accurately provides measurements of the buffer solutions to obtain the specified pH for the mobile phase. Transferring the values of an empirical table between laboratories can be challenging as a result of the many possible variables in the process of preparing and testing the stock solutions. The variables in an empirical table are the same as in any buffer preparation.
The sources of inaccuracy and variation included are the concentration of the stock solutions, the pH meter and electrode maintenance, the pH meter calibration, the individual technique, and the details of the buffer preparation. An empirical table protocol that reduces the variables was used to collect data from multiple independent laboratory trials. These trials were the basis for the empirical tables provided in Waters Driver Pack 4 Supplemental Release 1 (DWNL134853070).
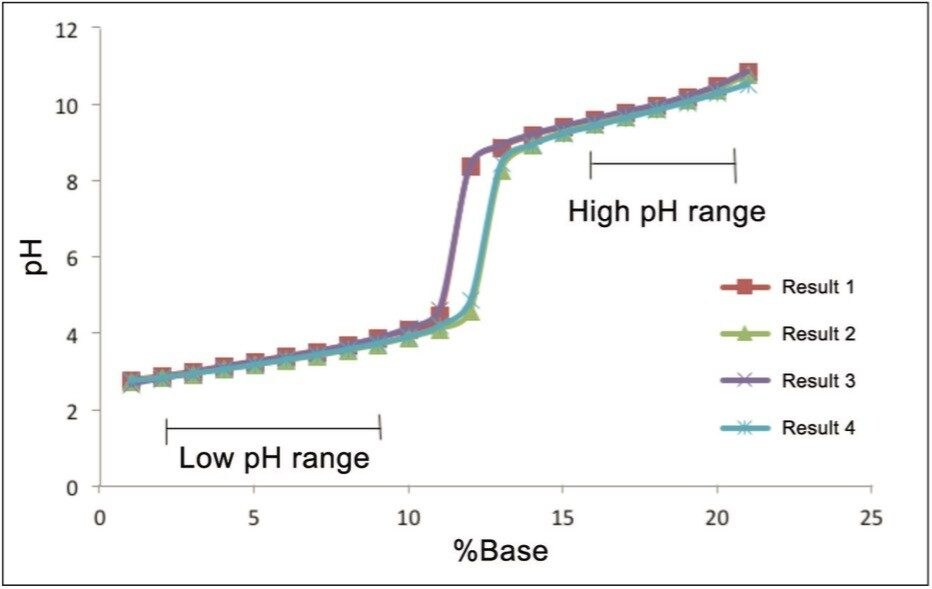
A set of data for an empirical table was collected by four independent scientists in which each scientist was given the same detailed empirical table protocol. The protocol manages some of the variability in the preparation of an empirical table. The protocol controls the variables of the stock solutions by providing part numbers for all required reagents as well as specific directions on how to make the stock solutions. Specifying calibration and operation as well as incorporating check samples throughout the experiment the protocol helps to manage the variables of the pH meter. The results from the reliability test are summarized in Table 1.
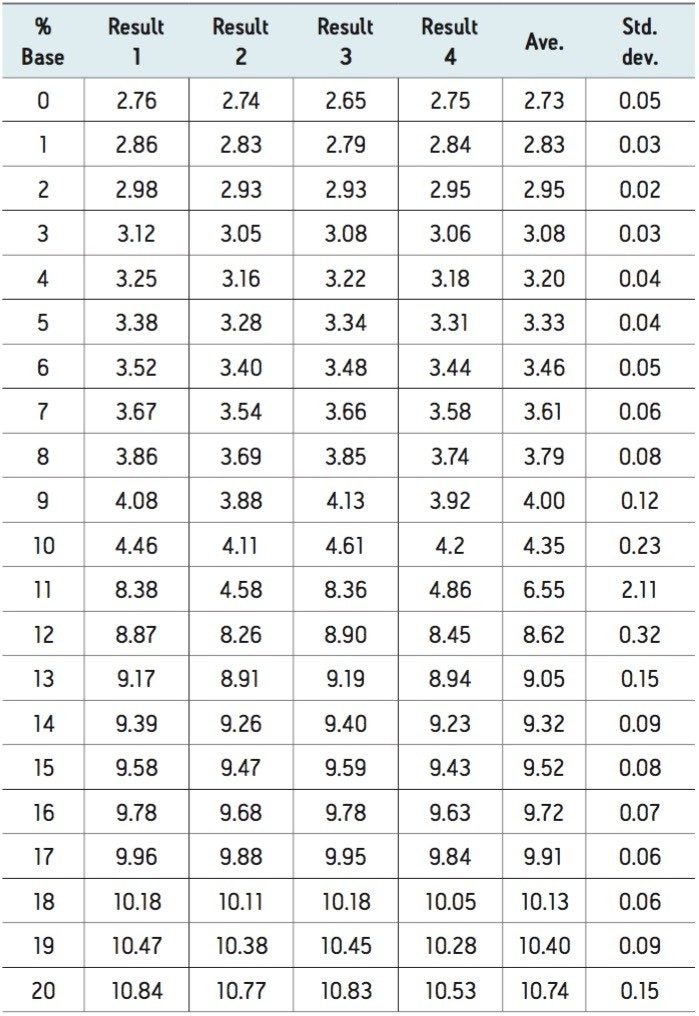
Good agreement among four independent trials and typically a 1% increase in the acid or base is in the order of approximately 0.1 pH changes. An increase in the proportion of acid or base leads to an expected change in pH that follows the logarithmic profile expected. The variability among laboratories is minimal where the buffering capacity is maximal and the range of values is consistent with the requirements of chromatographic selectivity. For most of the data points the standard deviation is quite low. The regions of the experiment where we expected the buffers to be at a low capacity, the standard deviation is quite high. The standard deviation is worse in the high pH range, but it is not outside of the useable range.
The data demonstrates that different ranges of pH are better controlled and more robust than other regions. There are two regions of the data set that provides more stability and covers a low range and a high range of pH values. In this example the low range is buffered by formic acid and the high region is buffered by ammonia. Within these ranges the standard deviation is within +0.10 and should be considered the useable range for two different buffer systems. The results from the reliability test demonstrate that it is important to select a reliable pH range for analysis since reliable chromatography outside of the pH ranges robust selectivity is not possible. Using a predictable and reliable range of the pH values ensures more reproducible and robust results.
The detailed protocol allows an empirical table to be transferred among laboratories. The results obtained are as expected from the titration of a buffering ion. Therefore, if the protocol is followed exactly, it is reasonable to use the average values collected from the reliability test in the empirical table for the two buffer systems. These two buffer system are included in Waters Driver Pack 4 Supplemental Release 1 (DWNL134853070).
The defined protocol provides two buffer systems, one low pH range and one high pH range, for the specified components of formic acid and ammonia. The two buffer systems can be used for LC-MS analysis if the buffer solutions are prepared exactly as the protocol states. The values observed across multiple laboratories are consistent. Therefore, the values can be used for the two specified buffer systems with the caveat that the stock solutions are prepared exactly as the protocol states. The protocol is reliable in controlling the variability associated with an empirical table and provides the ability to create an empirical table across multiple laboratories.
720005568, December 2015