This application note demonstrates traditional peptide isolation performed by HPLC with UV detection is easily accomplished using the Prep 150 LC, a simple and robust chromatography system. While crude peptide analysis was performed on an Alliance HPLC System configured with a 2998 Photodiode Array Detector, the isolation of the target peptide was predictable and reproducible when the method was transferred to the Prep 150 LC System.
Peptides are becoming more prevalent as diagnostics as well as therapeutics, mainly because of the technological advances in analytical methodology that have been made in recent years.1 Analytical methods run on state-of-the-art instrumentation show improved sensitivity and higher resolution in a shorter amount of time, all of which lead to increased throughput. With higher efficacy, safety, and tolerability in humans as compared to small molecules, and with lower production complexity and cost than protein-based biopharmaceuticals, peptides are increasingly more attractive as potential drug candidates.2 Whether peptides are made in solution, with solid phase strategies, or isolated from naturally-occurring sources, these potential products all require purification. The Prep 150 LC System is ideally suited for the isolation and purification of peptides with its reliable and robust instrumentation controlled by ChromScope Software. Although the crude peptide is commonly evaluated on an analytical HPLC system, with the knowledge of basic scaling principles, the larger scale isolation is straightforward and predictable with the Prep 150 LC System. Here, we demonstrate the analysis and UV-directed purification of a synthetic peptide using an Alliance HPLC System for screening and a Prep 150 LC System for isolation. This flexible strategy is applicable to many different types of samples which require purification. Methods can easily be optimized to save time and production cost.
|
Analytical columns: |
XBridge C18, 4.6 x 100 mm, 5 μm |
|
Analytical flow rate: |
1.46 mL/min |
|
Prep column: |
XBridge C18 OBD Prep, 19 x 100 mm, 5 μm |
|
Prep flow rate: |
25 mL/min |
|
Mobile phase A: |
0.1% trifluoroacetic acid in water |
|
Mobile phase B: |
0.1% trifluoroacetic acid in acetonitrile |
|
Wavelength: |
220 nm, 280 nm |
|
Gradients and injection volumes: |
as noted in figures |
|
Sample: |
Crude synthetic peptide comprised of the following 20 amino acid residues:7 polar, 8 nonpolar, 3 acidic, and 2 basic; 75% purity by HPLC |
|
Analytical: |
Alliance HPLC System, 2998 PDA Detector |
|
Prep: |
Prep 150 LC System configured with a 2545 Binary Gradient Module, 2489 UV/Visible Detector, Prep Inject Module, and Waters Fraction Collector III controlled by ChromScope v1.4.1 |


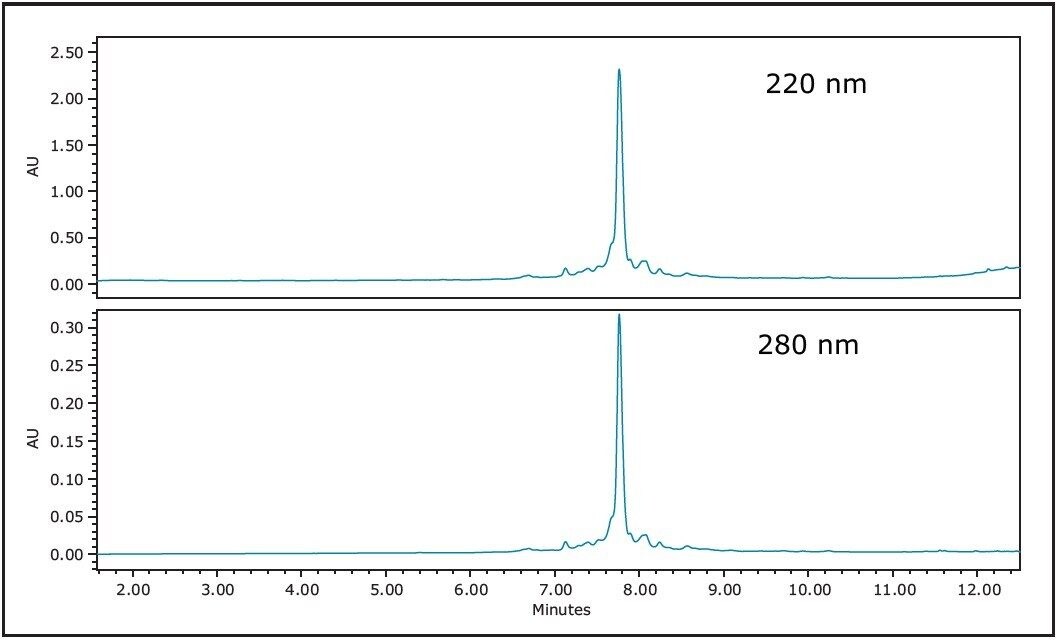
Solid phase peptide synthesis3 is a step-wise process where sidechain protected amino acids are sequentially added to a sequence by the formation of a peptide bond. Repeated cycles of coupling and deprotection with the addition of each amino acid continue until the desired sequence is assembled. After synthesis, the peptide is cleaved from the solid support and the side chain protecting groups are removed. As with any chemical reaction, incomplete deprotections, amino acid couplings, inadequate washing, or adduct formation during cleavage all contribute to impurity generation. Once the crude peptide is cleaved from the solid support and dried, it is analyzed and purified. In the present study, 14.4 mg of crude synthetic peptide was dissolved in 2 mL dimethylsulfoxide (DMSO) and filtered to give a final sample concentration of 7.2 mg/mL. HPLC analysis of the crude peptide on a 4.6 x 100 mm XBridge C18 Column showed that the purity was approximately 75% (Figure 1). The fast screening gradient ran from 5–50%B in 10 minutes, with a slope of 3.4% change per column volume (3.4%/cv). The peptide product eluted at approximately 33.5%B. Close examination of the chromatogram revealed the presence of several impurities eluting very closely to the main product peak.
As a general rule for focusing gradients,4 one-fifth of the screening slope usually slows the rate of change of the gradient to effectively move closely-eluting peaks away from the desired target peak. As shown in Figure 2, one-fifth the rate of change at 0.67%/cv improved the chromatographic profile, but an even shallower slope of 0.33%/cv further increased the resolution between the large target peak and the two peaks eluting on either side of the product. Improved resolution ultimately leads to higher column loading and/or better product purity. For this reason, the focused gradient with the more shallow slope was selected for the isolation of the peptide.
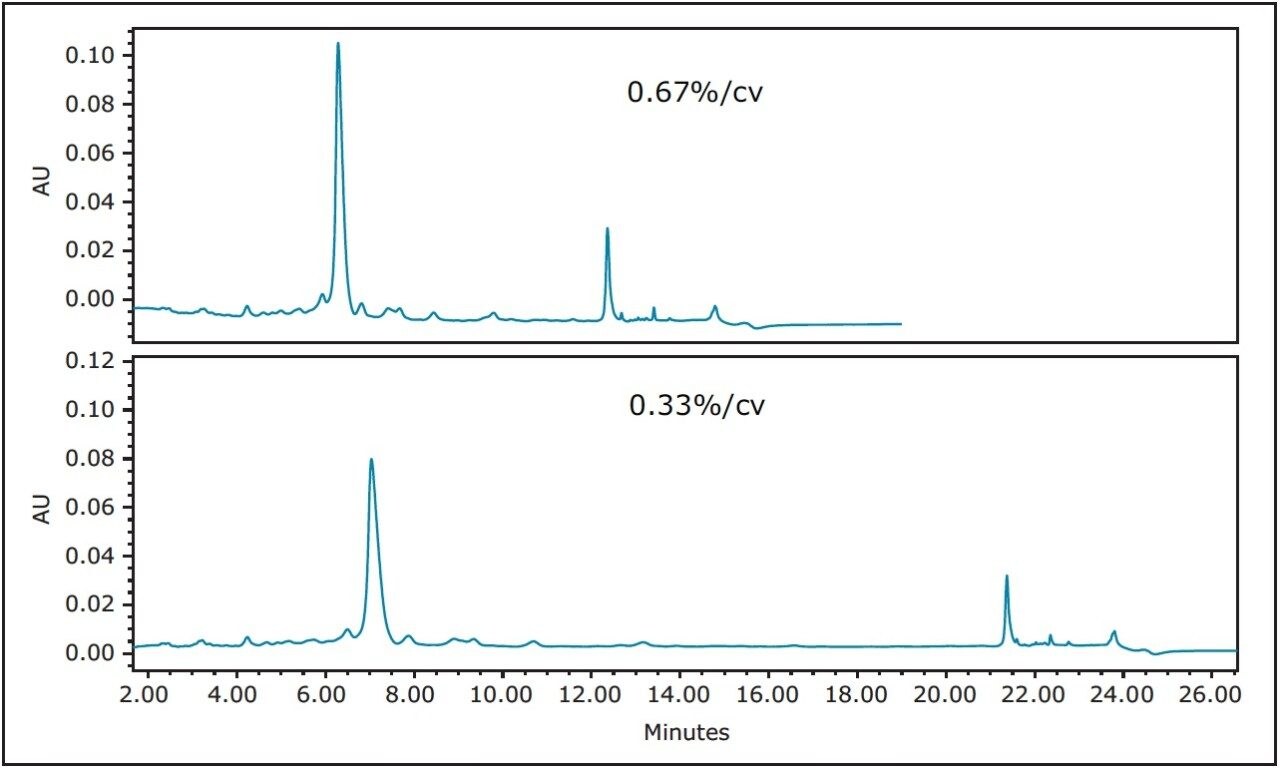
Figure 2. The effect of focusing the gradient used to analyze the crude peptide sample. Top trace: 28–36% B in 9 min, 5 μL. Bottom trace: 28–36%B in 18 min, 5 μL. Detection: 280 nm.
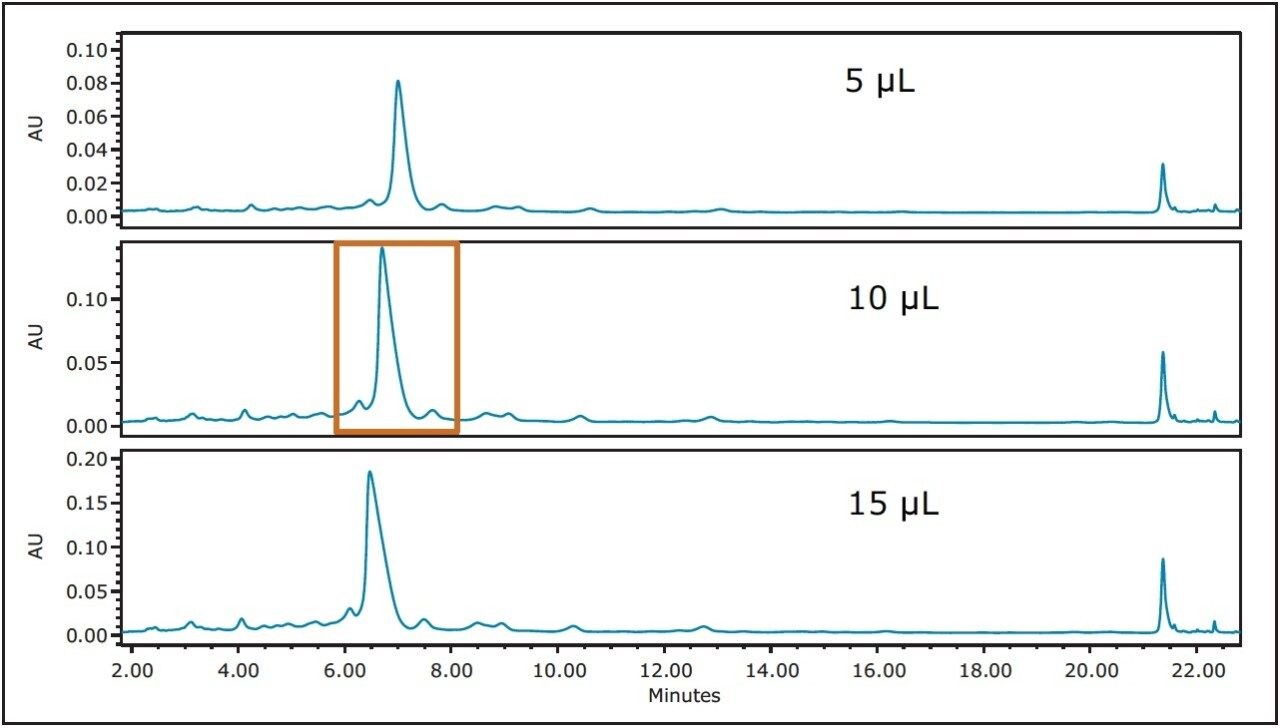
With the resolution improved to almost baseline with the very shallow 0.33%/cv focused gradient, a loading study was performed using the 4.6 x 100 mm analytical column (Figure 3). While a 15 μL injection on the 4.6 mm I.D. column still showed acceptable resolution, the more conservative 10 μL loading was chosen for subsequent scaling because the distance between the main peak and the two by-products was greater. Note that as the injection volume increased, the peptide peak eluted slightly earlier, a normal phenomenon commonly observed with increased column loading.
Proper scaling5 ensures that the chromatographic profile of a sample at the preparative scale will be identical to the chromatography obtained at the analytical scale. Geometric scaling of the flow rate and gradient to the 19 x 100 mm preparative column on the Prep 150 LC System showed the same chromatographic profile, as predicted (Figure 4). 85 microliters was injected on the prep column for the first run, equivalent to scaling from 5 μL on the analytical scale, for a more conservative approach toward determining the collection parameters.
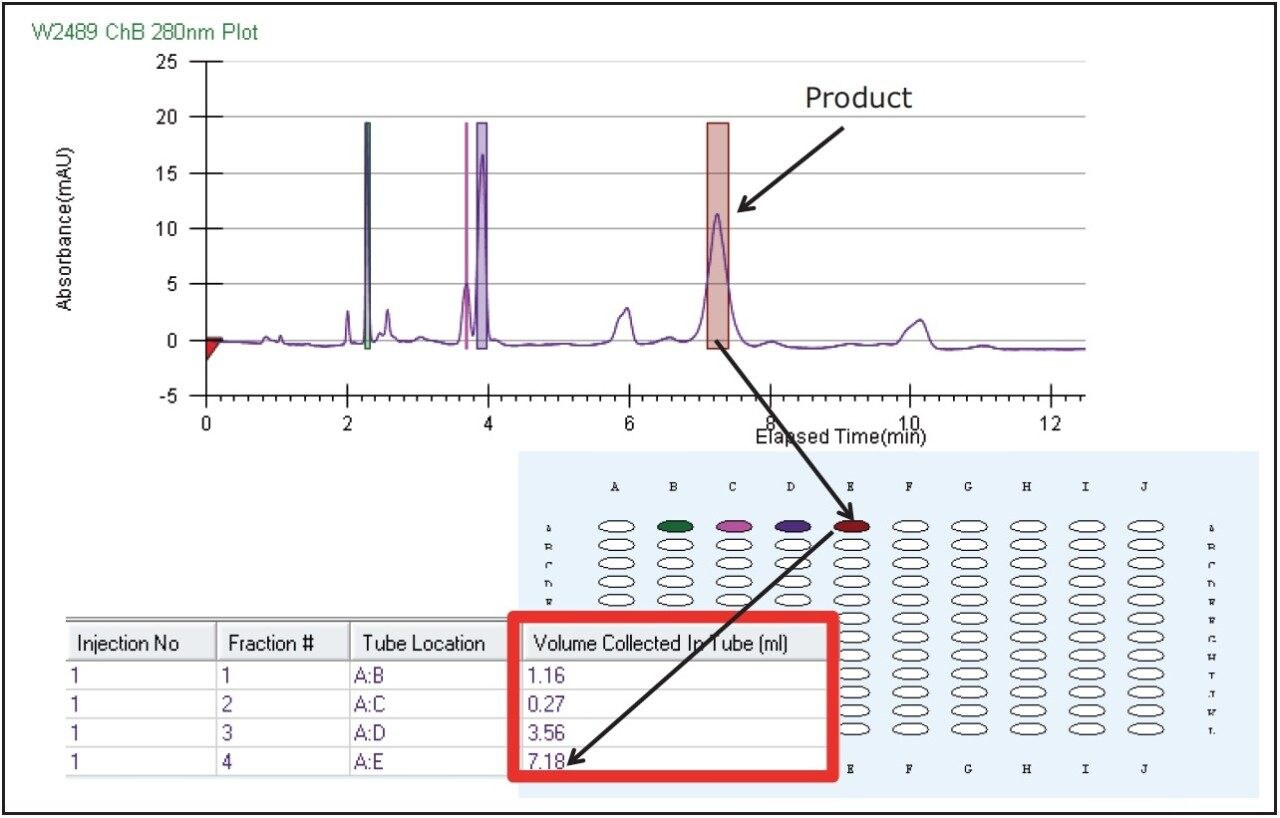
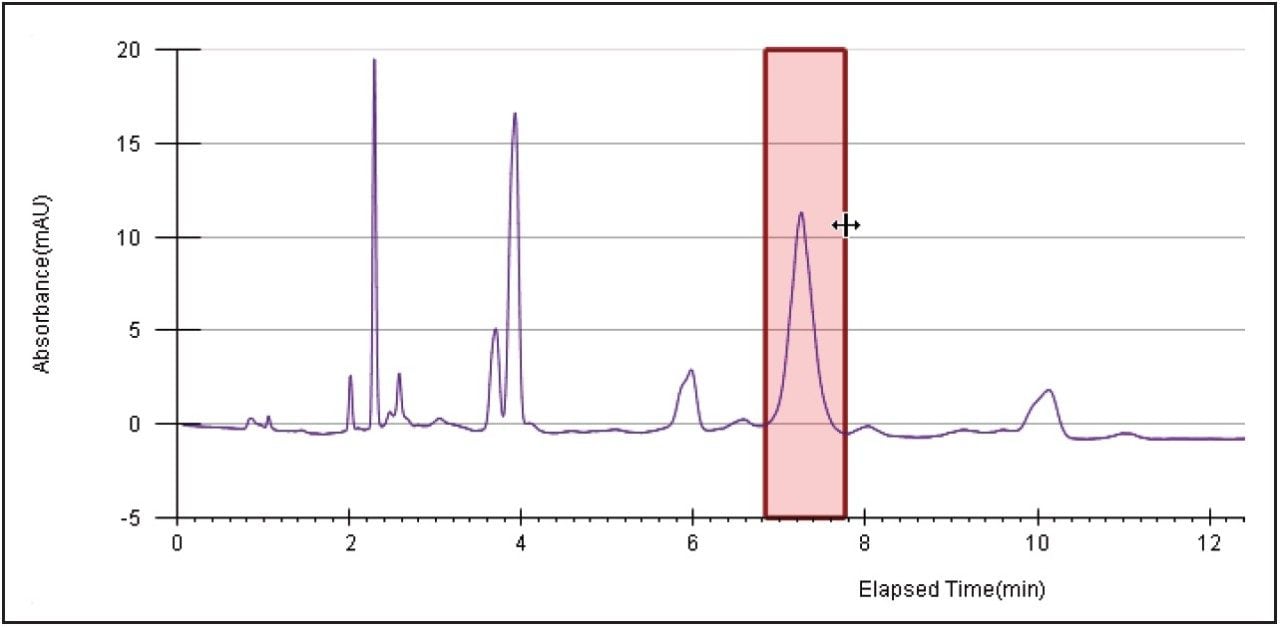
Three extra peaks were collected before the product peak eluted due to a wide collection window set in the method to ensure collection of the desired peptide. Although the peptide product was collected, a high threshold setting very conservatively heartcut the product peak. Fraction tracking was clearly visible with collection bed tube location and volume readily available in the ChromScope display. The fraction simulation tool in the software was used to reset the collection parameters by visually adjusting the collection window and threshold (Figure 5) on the chromatogram. These optimized parameters were used for the subsequent prep isolations.
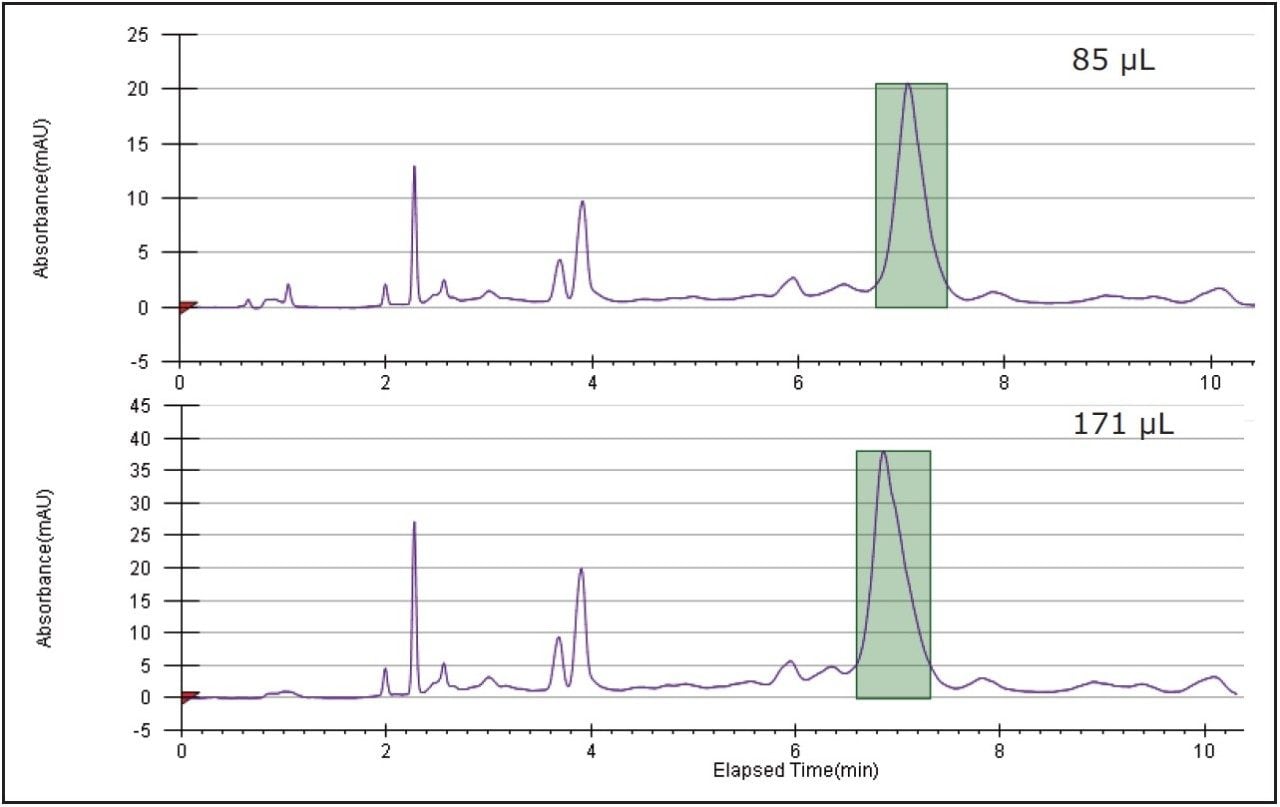
Two more preparative runs were performed, the first with an 85 μL injection to affirm the collection parameters, and the second with a 171 μL injection, the geometrically scaled injection volume as determined by the loading study on the 4.6 mm ID column. In the interest of saving time and solvent, the preparative runs were terminated after the target peak was collected and the column was manually washed and equilibrated. Figure 6 shows the chromatographic reproducibility of the prep runs, both of which were similar to the analytical profile obtained on the Alliance HPLC System. Knowledge of system volumes, identical column chemistry, and properly scaled injection volume, flow rate, and gradient time ensured predictable chromatography at the preparative scale.
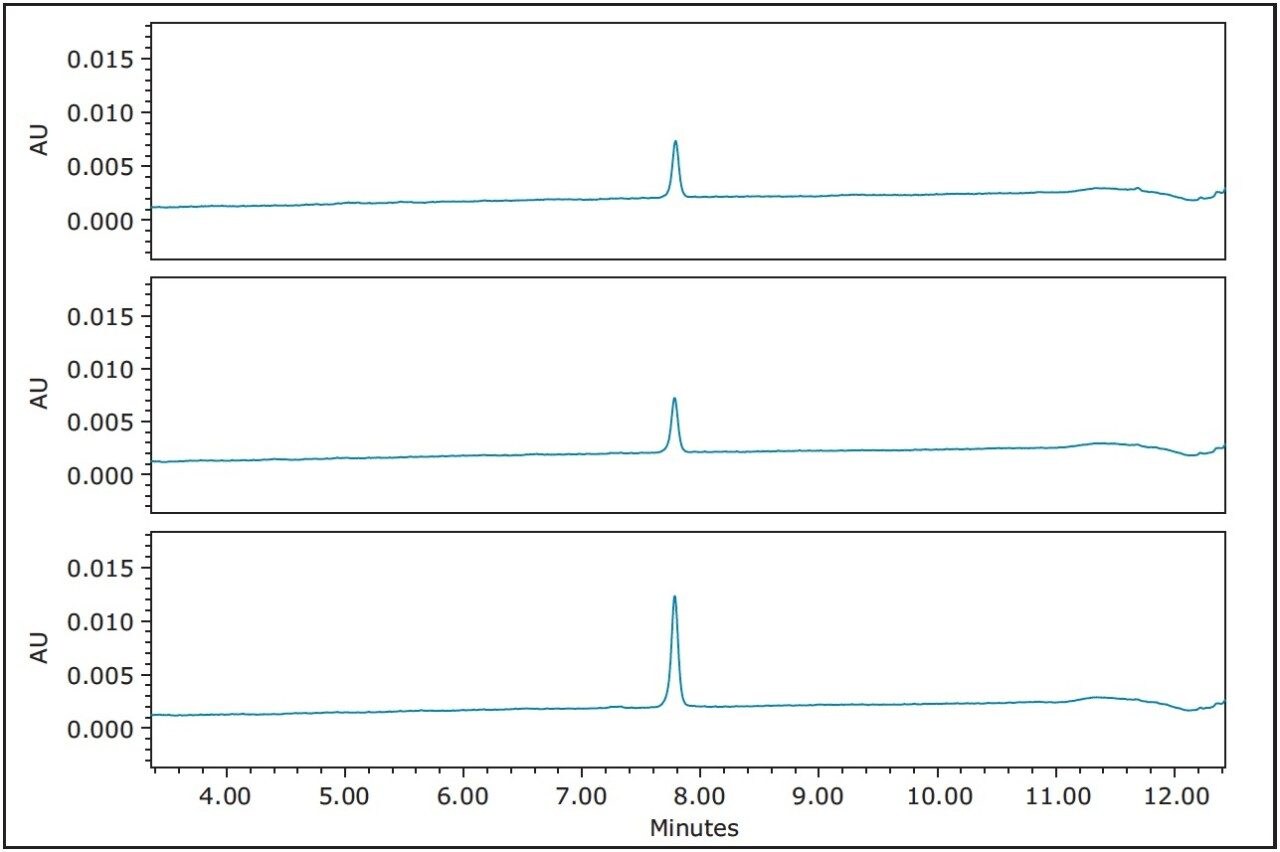
The fractions were analyzed on the 4.6 x 100 mm XBridge C18 Column immediately after collection using the original fast screening gradient. The fractions were very pure (Figure 7).
We have shown that traditional peptide isolation performed by HPLC with UV detection is easily accomplished using the Prep 150 LC, a simple and robust chromatography system. While crude peptide analysis was performed on an Alliance HPLC System configured with a 2998 Photodiode Array Detector, with knowledge of system volumes and basic preparative scaling rules, the isolation of the target peptide was predictable and reproducible when the method was transferred to the Prep 150 LC System. Focusing the gradient was useful in improving the quality of the separation by increasing the resolution between closely-eluting by-products. ChromScope Software, with its intuitive and easy-to-use preparative features like fraction simulation, made setting collection parameters fast and uncomplicated. Fraction tracking information, including collection volume, was unambiguous with collection tubes clearly marked on the chromatogram and on the collection bed.
720005455, July 2015