
This application note describes the use of the ACQUITY UPLC System, coupled with the Xevo TQ-S Mass Spectrometer for the determination of three common beta agonist residues (clenbuterol, ractopamine, and salbutamol) in meat.
Compared to the current GB/T 22286-2008 LC method, this UPLC-MS/MS method provides a more simplified sample preparation procedure, 73% shorter run times (7 minutes versus 26 minutes per run with the GB method), uses less solvent, and saved the equivalent of 1,740 hours of instrument time.
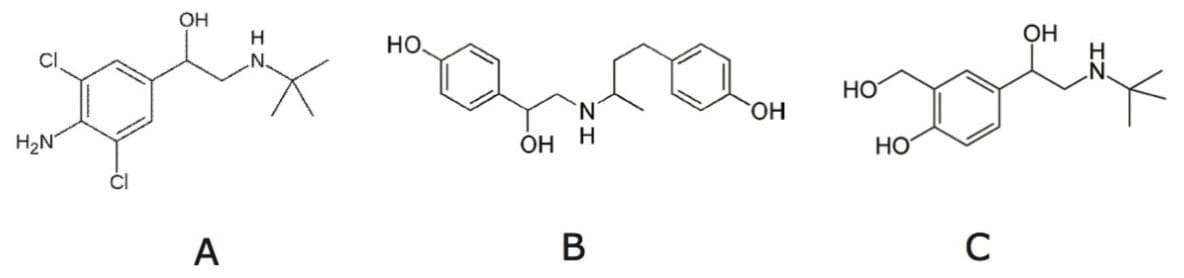
Beta-agonists, or β2-adrenergic agonists are, often used as drugs to treat asthma and other pulmonary diseases. They act on the β2-adrenergic receptor and cause smooth muscle relaxation, resulting in the dilation of bronchial passages and other effects. However, some beta agonists have been used illegally in meat husbandry, since they can also promote muscle growth instead of fat growth in animals, thereby increasing weight gain, enlargement of the ribeye area, and, therefore, additional total red meat yield. Consumption of beta-agonist contaminated meat has caused food poisoning incidents, and many countries and regions have banned its use for meat production.1,2 Clenbuterol, ractopamine, and salbutamol are commonly used beta-agonists. In China, clenbuterol, salbutamol, and ractopamine are prohibited for use in food-producing animals,3,4 and their presence in meat products is illegal. Figure 1 shows these three compound structures.
Liquid Chromatography coupled with tandem Mass Spectrometry (LC-MS/MS) has been adopted officially in China for the determination of beta-agonist residues in meat products.5 Using this method (GB/T 22286-2008), samples undergo enzymatic hydrolysis, protein precipitation, extraction, an SPE clean-up procedure, and then they are separated using reversed-phase liquid chromatography on a C18 column and detected by tandem mass spectrometry. Stable isotope internal standards (clenbuterol-d9 and salbutamol-d3) are used for quantitative analysis. The typical LC-MS/MS analysis run time for this method is 26 minutes.
Since the first commercialization of UltraPerformance Liquid Chromatography (UPLC) by Waters in 2004, the benefits of UPLC over traditional HPLC have been well documented, and UPLC has been widely applied to many analytical areas. This application note describes the use of the ACQUITY UPLC System, coupled with the Xevo TQ-S Mass Spectrometer for the determination of three common beta agonist residues (clenbuterol, ractopamine, and salbutamol) in meat. Compared to the GB method,5 the sample run time has been shortened from 26 minutes to 7 minutes with UPLC; the sample extraction procedure and chromatographic conditions have been optimized; and additional MRM transitions and stable isotope international standards have been employed to improve the analysis. In addition, the ACQUITY UPLC HSS T3 1.8 µm Column was investigated during the actual meat product analysis.
|
LC System: |
ACQUITY UPLC |
|
Runtime: |
7.0 min |
|
Column: |
ACQUITY UPLC HSS T3 1.8 μm, 2.1 x 100 mm |
|
Column temp.: |
30 °C |
|
Injector temp.: |
10 °C |
|
Injection volume: |
5 μL |
|
Flow rate: |
0.4 mL/min |
|
A: |
0.1% (v/v) formic acid aqueous solution |
|
B: |
0.1% (v/v) formic acid in acetonitrile |
|
Time |
Flow |
A% |
B% |
Curve |
|---|---|---|---|---|
|
Initial |
0.4 |
95 |
5 |
Initial |
|
1.2 |
0.4 |
95 |
5 |
1 |
|
2.5 |
0.4 |
59 |
41 |
6 |
|
3.5 |
0.4 |
59 |
41 |
1 |
|
3.7 |
0.4 |
0 |
100 |
6 |
|
4.8 |
0.4 |
0 |
100 |
1 |
|
5.0 |
0.4 |
95 |
5 |
6 |
|
MS System: |
Xevo TQ-S MS |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
3.5 kV |
|
Cone voltage: |
30 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
900 L/H |
|
Collision gas flow: |
0.19 mL/min |
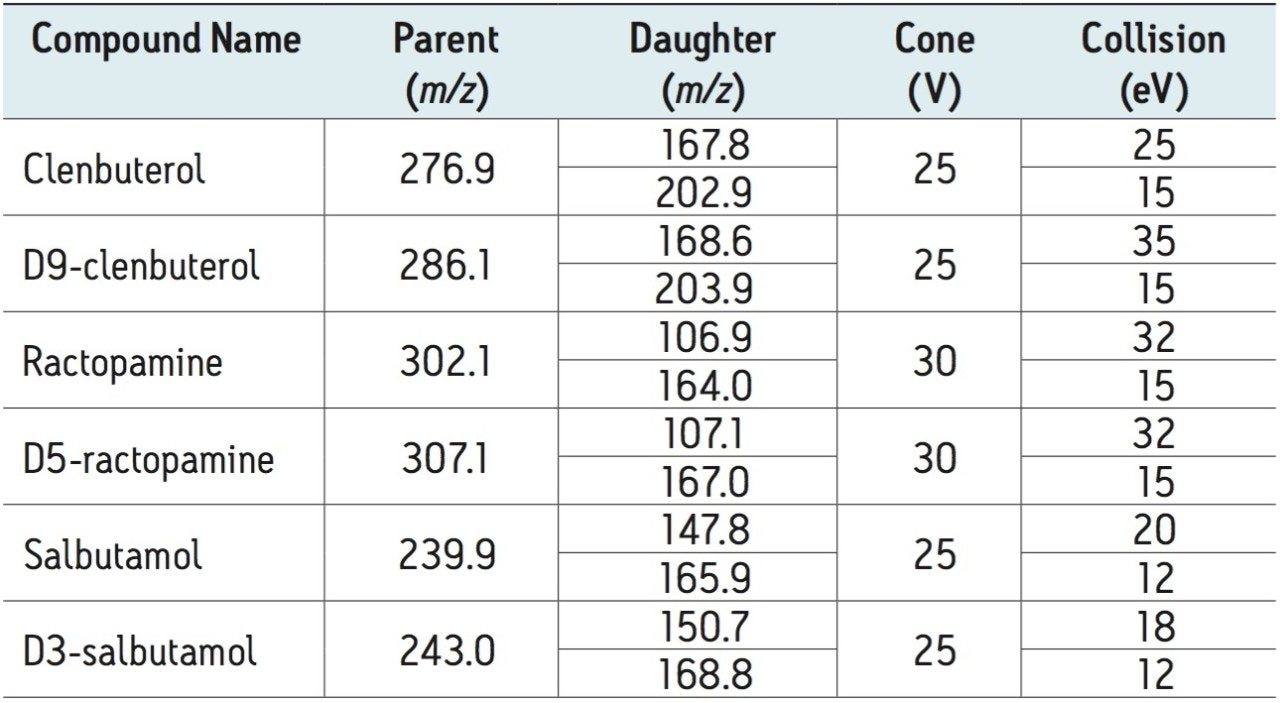
Diagnostic MRM transitions (see Table 1) were generated using Waters IntelliStart Software.
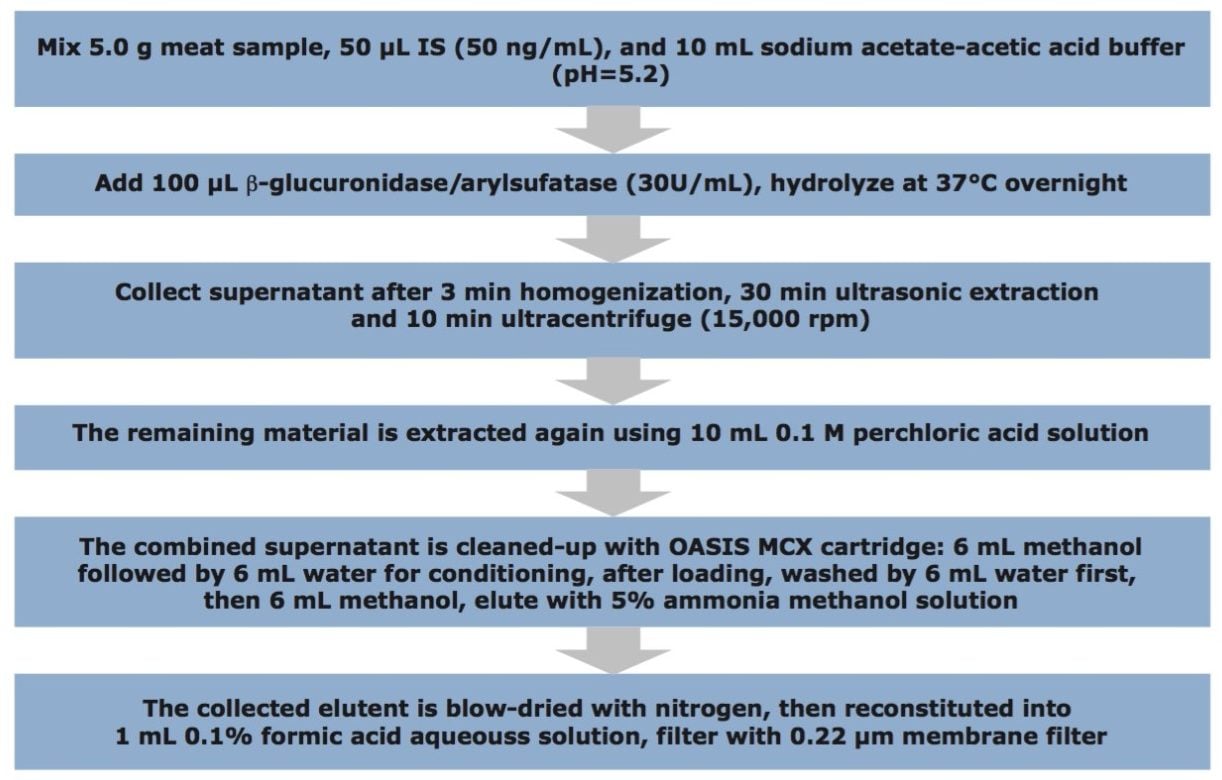
Overview of the sample preparation is provided in Figure 2.
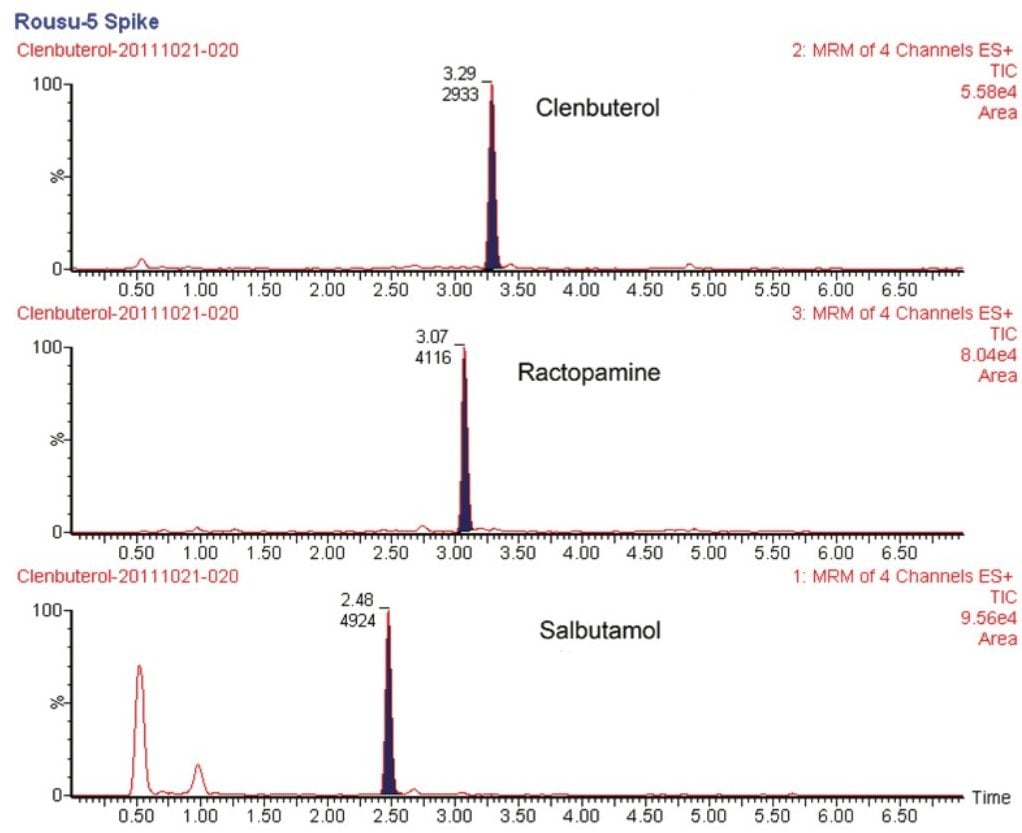
The elution of clenbuterol, ractopamine, and salbutamol was achieved by a gradient elution on an ACCUITY UPLC HSS T3 Column. The retention times of these compounds were 3.29, 3.08, and 2.49 min, respectively. The identification of these compounds was confirmed by their corresponding MRM transitions and by their retention times using UPLC separation with standard solutions and spiked samples. Two MRM transitions from precursor ions to product ions were used in this method, as compared to only one MRM transition employed in the official (GB/T 22286-2008) method. This provided additional confidence in the analysis results. Stable isotope internal standards were used to correct any variation during sample preparation and ionization for sample quantification (standard working curves not shown). Typical chromatograms of samples with and without spiked analytes are shown in Figures 3 and 4.
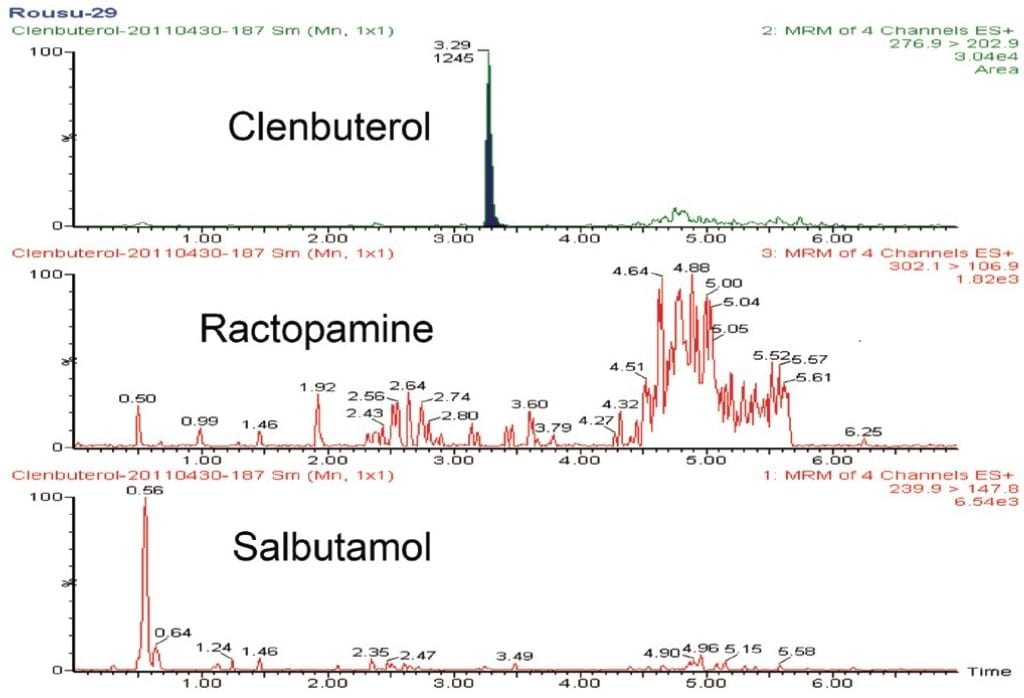
The recovery study was conducted at three concentration levels (0.1, 1.0, and 10.0 μg/kg meat sample, as shown in Table 2). The recoveries for all three levels ranged from about 80% to 100% with relative standard deviations less than 10.5%. The limit of detection (LOD) for this method is estimated at 0.02 μg/kg, and the limit of quantitation (LOQ) is 0.05 μg/kg. This LOD is about 1/25th of the current GB T 22286-2008 method’s LOD (0.5 μg/kg).

This method has been applied to the analysis of 140 meat samples obtained in local stores. Out of the 140 meat products that were tested, 39% of them contained up to 4.8 µg/kg of clenbuterol (0.2 to 4.8 µg/kg). Neither ractopamine nor salbutamol was found in any samples (Data not shown).
Some food analysis labs are hesitant to adopt UPLC Technology due to concerns regarding the lifetime of the UPLC column. During our analysis of beta agonists in meat products, and other food-related analysis using UPLC columns, when proper care is given to the system operation, and the preparation and handling of the mobile phases and samples,6 we did not observe any column reliability issues. Figure 5 shows a comparison of the ACQUITY UPLC HSS T3 Column performance in our routine analysis of beta agonists residue in meat products. When the chromatograms collected on the same column after its 1,600th injection and its 5,500th injection were compared, no discernable differences in peak shape and retention times were observed. The ACQUITY UPLC HSS T3 Column proved to be an extremely reliable and robust column for use in food testing applications.
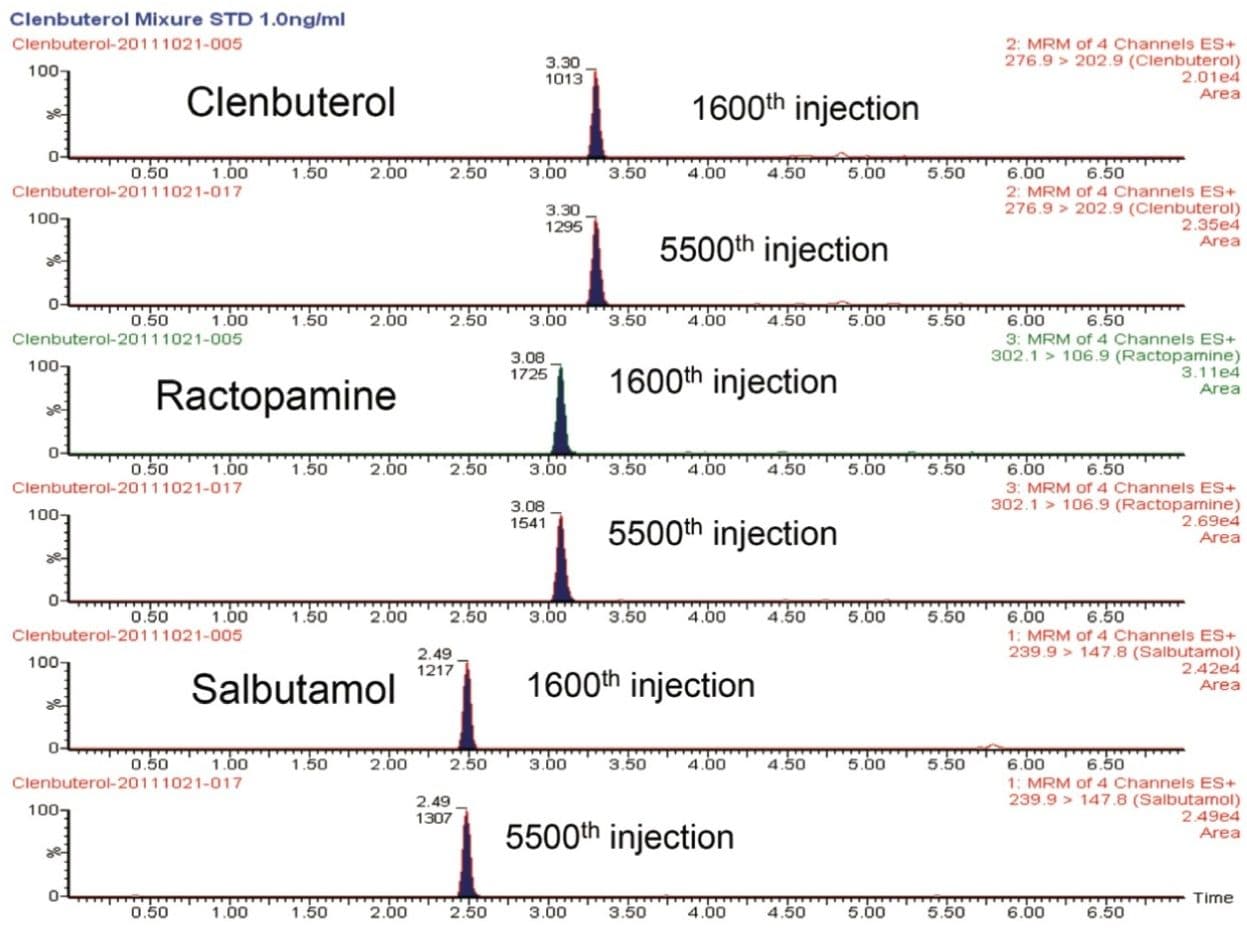
In addition to this ACQUITY UPLC HSS T3 Column, our experience with other UPLC columns, such as the UPLC BEH 300 and the ACQUITY UPLC BEH C18, also showed they are very reliable columns. With proper column and system care, these columns still deliver consistent column efficiency after 5,000 injections in food testing applications. Data not shown.
720004388, June 2012