This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrate that isobaric oligosaccharide mixtures can be resolved and identified using ion mobility TOF mass spectrometry with the MALDI SYNAPT G2 HDMS System.
Ion mobility mass spectrometry is a rapid and efficient technique for differentiating structural isomers of oligosaccharides.
Glycosylation plays a vital role in stability, in vivo activity, solubility, serum half-life, and immunogenicity of many recombinant therapeutic proteins. Because therapeutic glycoproteins are typically manufactured using non-human expression cell systems, the glycoforms from recombinant proteins and from human sources can be very different.
For example, glycosylation can change significantly under poorly-controlled culture conditions, making it a key indicator of process robustness. It is therefore essential to demonstrate that glycosylation is consistent, showing control over the production process, and to establish acceptable variation limits for biotherapeutic production.
Oligosaccharides (sugars) from glycoproteins frequently exist as sets of isomers, where differences in glycosidic linkage or in position isomeriation are present. These apparently minor differences may significantly impact the safety and efficacy profile of a biotherapeutic. Organizations that fail to comprehensively profile the glycans may risk expensive and damaging product recalls or difficulties with regulatory approval. Therefore a comprehensive characterization of the glycan profile is required to clarify the variation in these structural and spatial isomers.
Oligosaccharide standards difucosyl-p-lacto-Nhexaose II and difucosyllacto-N-hexaose-a (V-labs Inc.) were used. Each oligosaccharide standard was dissolved in water (0.2 mg/mL) and was mixed at 1:1 ratio to generate a sample containing both isobaric oligosaccharides. The individual and mixture samples were mixed 1:1 (v/v) with a 2,5-dihydroxybenzoinc acid (DHB) MALDI matrix solution (20 mg/mL in ethanol), respectively, before spotting onto a stainless steel MALDI target plate. All samples were analyzed in positive ion MS and MS/MS mode, and MS signals reported are from the sodiated adducts (MNa+: 1365.4278). Data processing was accomplished using either MassLynx 4.1 or DriftScope 2.1 software, which supports data viewing, processing, and collisional cross section measurements for ion mobility mass spectrometric analysis.
Collisional cross-sections of each oligosaccharide were automatically calculated after the system was calibrated using a polyalanine calibration standard.
The unique ability of the SYNAPT G2 HDMS mass spectrometer to differentiate isobaric structural isomers of oligosaccharides is illustrated in Figure 1, where the drift time distributions for each of the oligosaccharides, as well as the oligosaccharide mixture are shown. The drift time distribution for the oligosaccharide mixture (Figure 1B) shows two distinct peaks with drift times of 8.80 and 9.56 ms, whereas the pure difucosyllacto-N-hexaose-a sample generates one mobility peak at 9.49 ms (Figure 1A) and difucosyl-p-lacto-N-hexaose II sample at 8.66 ms (Figure 1C). Thus, the ion mobility separation enables clear resolution and assignment of the two isomeric peaks.
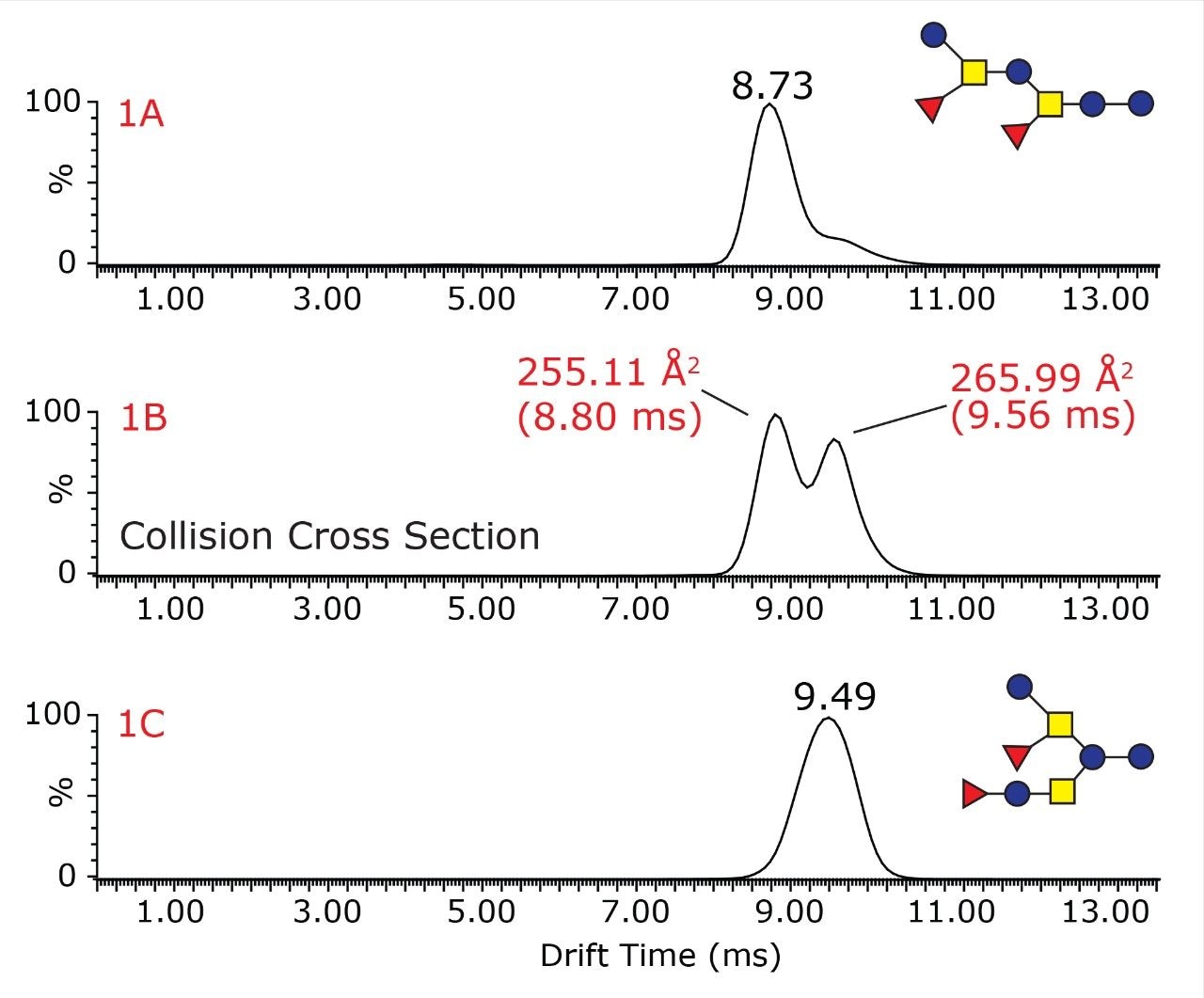
Overall, this figure demonstrates that travelling wave ion mobility can be used to rapidly separate isobaric oligosaccharides in mixtures submitted to MALDI IMS-TOF analysis.
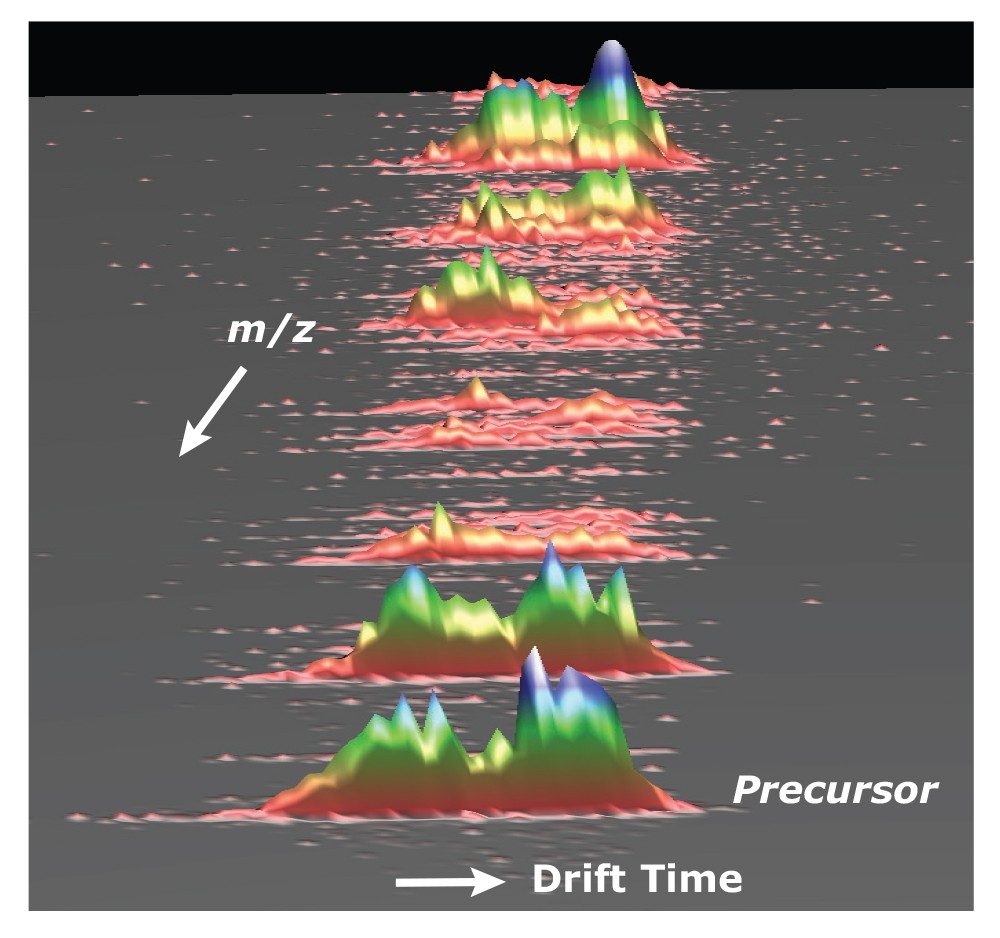
Fragmentation following ion mobility separation is further capability of the SYNAPT G2 HDMS platform that offers additional information for structural assignment confirmation. Because fragmentation can be performed following the ion mobility separation, the drift time of all of the attendant fragment ions will be conserved with the intact glycan precursor ions (Figure 2). Thus, multiple species can be simultaneously fragmented for further analysis. Enabling fragmentation to be performed both prior to and following the ion mobility separation (data not shown) further enhances the information content in the analysis of complex oligosaccharides.
Ion mobility mass spectrometry is a rapid and efficient technique for differentiating structural isomers of oligosaccharides. The Triwave Technology of the SYNAPT G2 HDMS offers enhanced functionality by performing fragmentation either before and/or after ion mobility separation to elucidate the complex structures of oligosaccharides. These oligonucleotides can be described not only by their mass or mass-to-charge but by their collisional cross-sectional areas. Organizations wishing to increase their productivity, protect their intellectual property, or improve the characterization of their complex biotherapeutic products will see immediate benefits by providing their scientists access to ion mobility-enabled mass spectrometry platforms.
720004022, July 2011