
This application note demonstrates one application of ACQUITY UPLC in the chemical analysis market that can impact everyday life.
Ballpoint pen inks are available in a rainbow of colors but when the intended application is signing a legal document, black ink is preferred. This is related to its superior photostability. Many dye components used to formulate inks degrade with exposure to light, which can result in a faded and legally indefensible signature.
Light exposure tests are typical for determining dye photostability. The sensitivitity and resolution of the Waters ACQUITY UltraPerformance LC (UPLC) System enables the determination of which dye in a mixture is degrading. Information about purity and composition of dyes for product development, lot to lot product qualification and competitive product deformulation is also needed and readily obtained.
This application note describes the analysis of photodegraded ink extracts and triarylmethane dyes (1– 3) standards and the ACQUITY UPLC Sytem with UV and single quadrupole MS detection. Information about the photostability of individual ink dyes was obtainable by analyzing extracts from written samples via UPLC of separated dye components. The previous application note described the 1 minute separation with UPLC and UV detection and benefits relative to the typical 20 minute HPLC run time.1-5

|
Sample Preparation4 |
|
|---|---|
|
Extraction solvent: |
60v% acetic acid (1.25%)/40v% CH3CN |
|
Dye standard solution: |
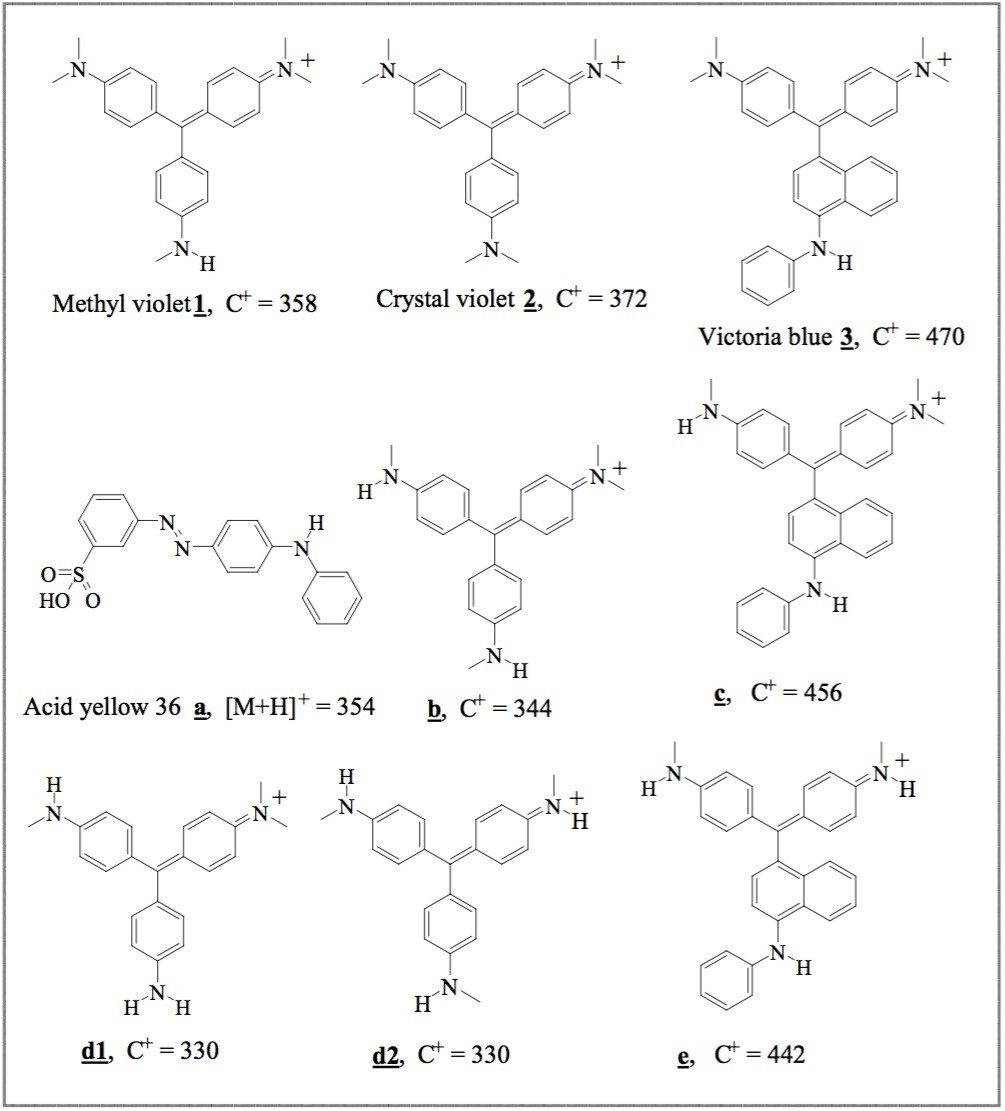
triarylmethane (1–3) dyes (Figure 1) were dissolved in extraction solvent (10 μg/mL). |
|
Ink extracts: |
Thirty lines (~1 cm long) were written with ballpoint pens on a 20lb XEROX multipurpose 4200 paper. For the photo-degradation study, the written samples were placed under a UV lamp for 3 to 24 hours. The fresh and photo-degraded written samples were cut and placed in vials with 0.5-1.5 mL of solvent. The vials were gently rotated for one hour until the paper showed no visible signs of ink. The ink extracts were used for UPLC analysis. |

|
System: |
ACQUITY UPLC with ACQUITY UV Detector and Waters ZQ 2000 Mass Detector |
|
Cloumn: |
ACQUITY UPLC BEH C18 2.1 x 50 mm |
|
Column temp: |
50 °C |
|
Weak wash: |
95:5 Water: CH3CN (500 μL) |
|
Strong wash: |
50:50 Water: CH3CN (300 μL) |
|
Seal wash: |
90:10 Water: CH3CN (5 min) |
|
Mobile phase A: |
10mM NH4OAc in 95v% H2O/5v% CH3CN |
|
Mobile phase B: |
10mM NH4OAc in 5v% H2O/95v% CH3CN |
|
Gradient: |
25% B to 80% B in one minute, curve 5 |
|
Flow rate: |
1 mL/min |
|
Injection: |
2-5 μL |
|
Detection: |
UV absorbance |
|
Sampleing rate: |
20 pts/s |
|
Filter response: |
0.1 s |
|
Probe: |
ES+ |
|
ES capillary (kV): |
3.2 |
|
Cone (V): |
40 |
|
Extractor (V): |
2 |
|
RF Lens (V): |
0.5 |
|
Source temp (°C): |
140 |
|
Desolvation Temp (°C): |
450 |
|
Cone Gas flow (L/Hr): |
50 |
|
Desolvation Gas (L/Hr): |
800 |
|
LM resolution: |
15 |
|
HM resolution: |
15 |
|
Ion energy: |
0.3 |
|
Multiplier: |
500 |
|
Scan range: |
150 to 500 Da |
|
Scan time: |
0.1 sec |
|
Inter-scan delay: |
0.05 sec |
Data acquistion and analyis was performed with Waters Empower Software.
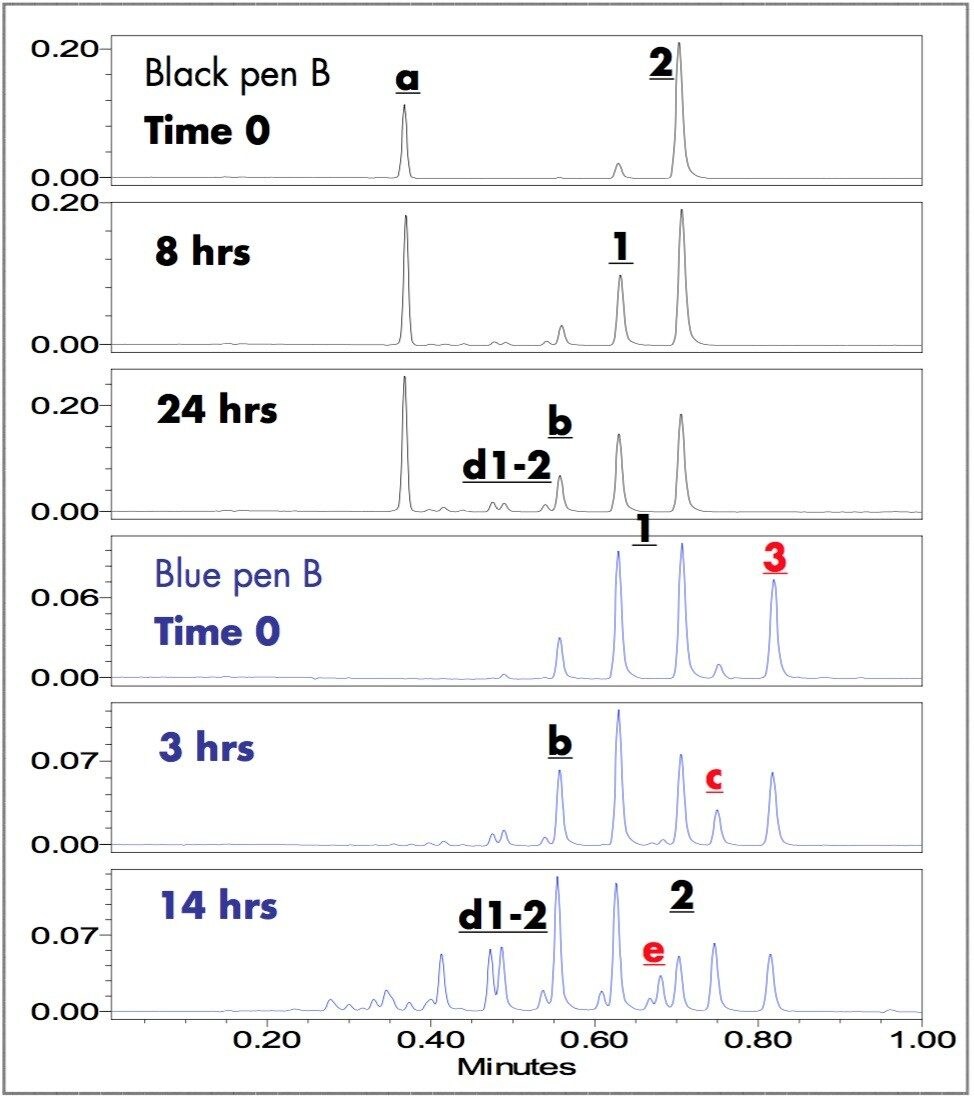
The triarylmethane dyes (1-3) and the components extracted from the samples written with black and blue ballpoint pen inks are easily separated in less than one minute using the ACQUITY UPLC System. Figure 2 shows the UV chromatograms of triarylmethane dye mixture (1-3) and the extracts of the fresh black and blue written samples.
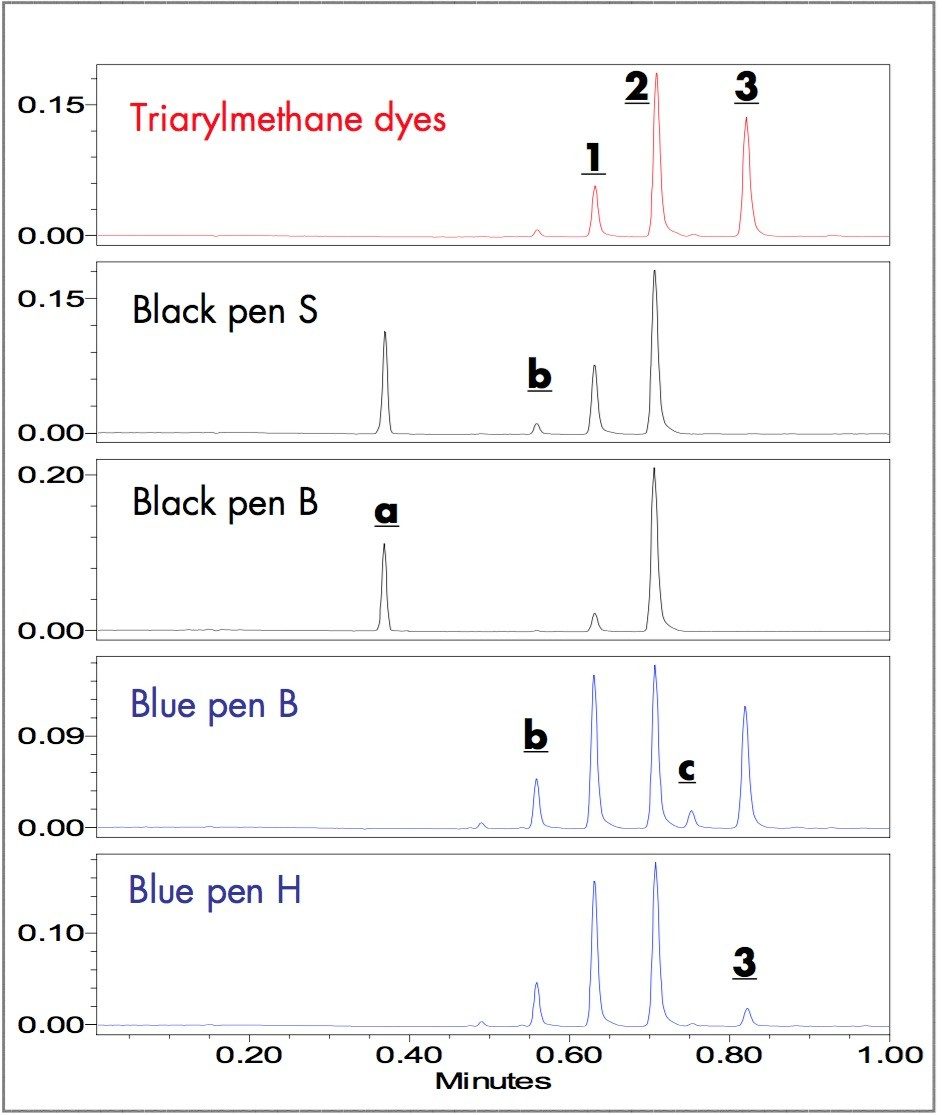
Five peaks were observed in the chromatogram of the triarylmethane dyes. By retention time matchng with the standards, the three major peaks at 0.63, 0.71, and 0.82 minutes are Methyl Violet (1), Crystal Violet (2) and Victoria Blue (3). Retention times of two impurity peaks are 0.56 (b) and 0.75 (c) minutes.
Black pen S has four peaks: two match retention times of 1 and 2; the other two peaks have retention times 0.37 (a) and 0.56 (b) minutes. Black pen B has three peaks: two match retention times of 1 and 2; the other has retention time 0.37 (a) minutes.
Blue pens B and H have five major dye components: three peaks match retention times of 1, 2 and 3; the other two peaks (b & c) have retention times 0.56 and 0.75 minutes. Although both blue pens have the same dye components, the relative amount of dyes in their formulas are significantly different. The relative peak intensities of c and 3 in blue pen B are significantly larger than those in blue pen H. When additional infomation is needed to track and indentify the dye peaks, the ZQ Mass Spectrometer in combination with UV detection is capable of providing the requisite data.
Figures 3 and 4 show the ES+ TIC (electrospray positive total ion current) chromatograms and the extracted mass spectra of major peaks. The ZQ Single Quadrupole Mass Spectrometer detected all the peaks observed by UV and some additional components. A small peak, b ,was detected in the TIC chromatogram of blue pen B and peak x, unique to Blue pen H, was detected at 0.88 minutes (m/z 304).
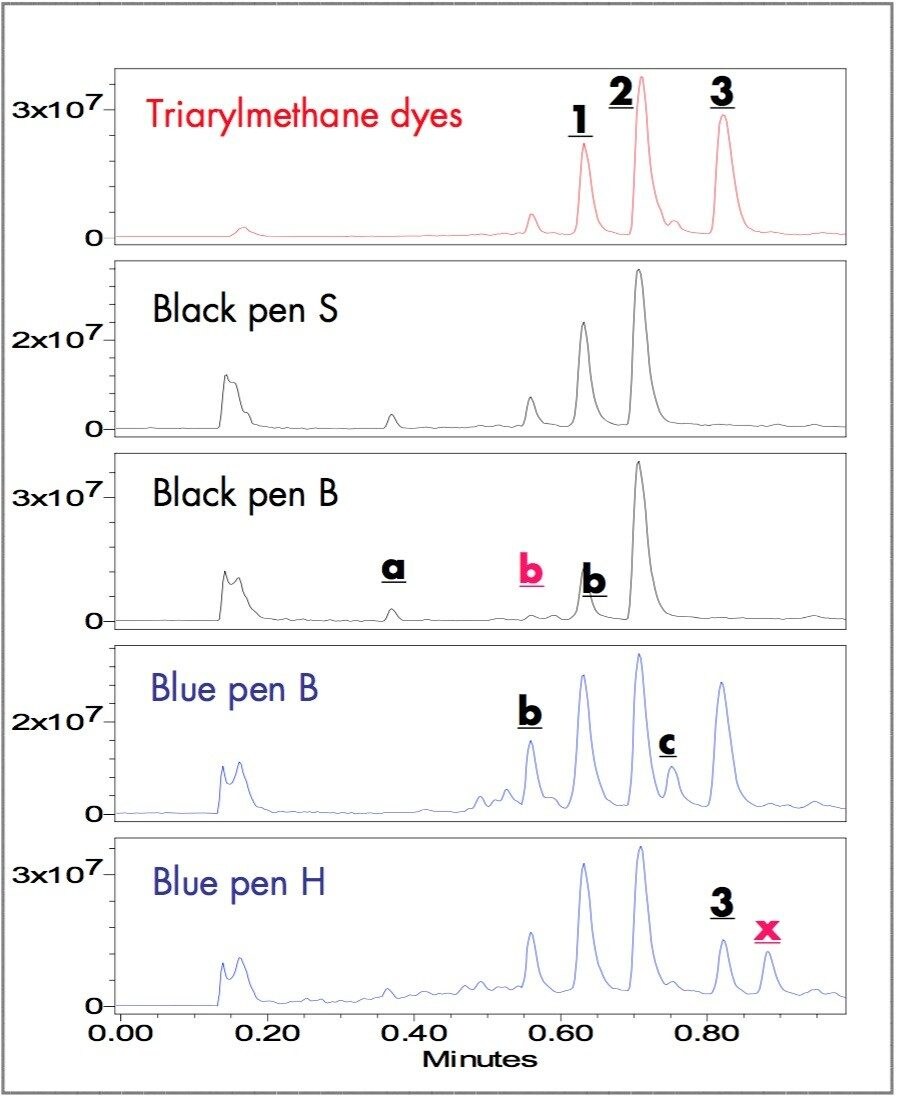
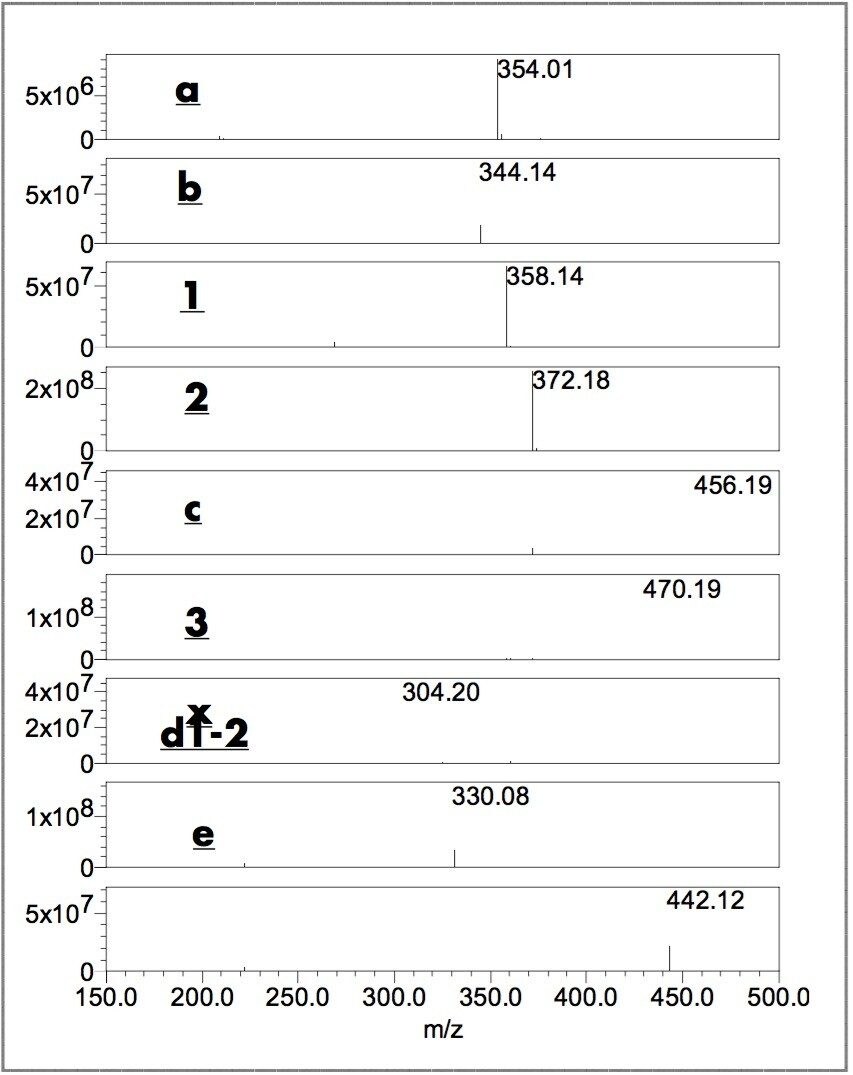
The m/z of peaks 1 (358 amu), 2 (372 amu) and 3 (456 amu) confirmed that Methyl Violet, Crystal Violet, and Victoria Blue are in the ballpoint pen ink dye formulas. The extracted mass spectra of peaks at 0.37 (a), 0.56 (b) and 0.75 (c) minutes with m/z 354, 344 and 456, respectively, suggest compounds a, b and c.5
Figure 5 and 6 show UV and TIC chromatograms of fresh and photo-degraded written samples. The relative peak intensity of d1-2, b, 1, e, and c rose over time while the peak intensities of 2 and 3 decreased. The m/z of the rising and decreasing components of each peaks were confirmed and identified by ZQ mass spectrometry (Figure 6). The photo-degradation peaks (e and d1-2) at 0.68, 0.49 and 0.47 minutes with m/z 442, 330, and 330, respectively, suggest compound e and isomeric compounds d1 and d2.5 In these experiments, the black written samples are more stable than the blue samples. Large amounts of photo-degradation products were observed in the blue samples after 14 hours of UV lamp exposure, whereas, relatively small amounts were found in the black samples after 24 hours.
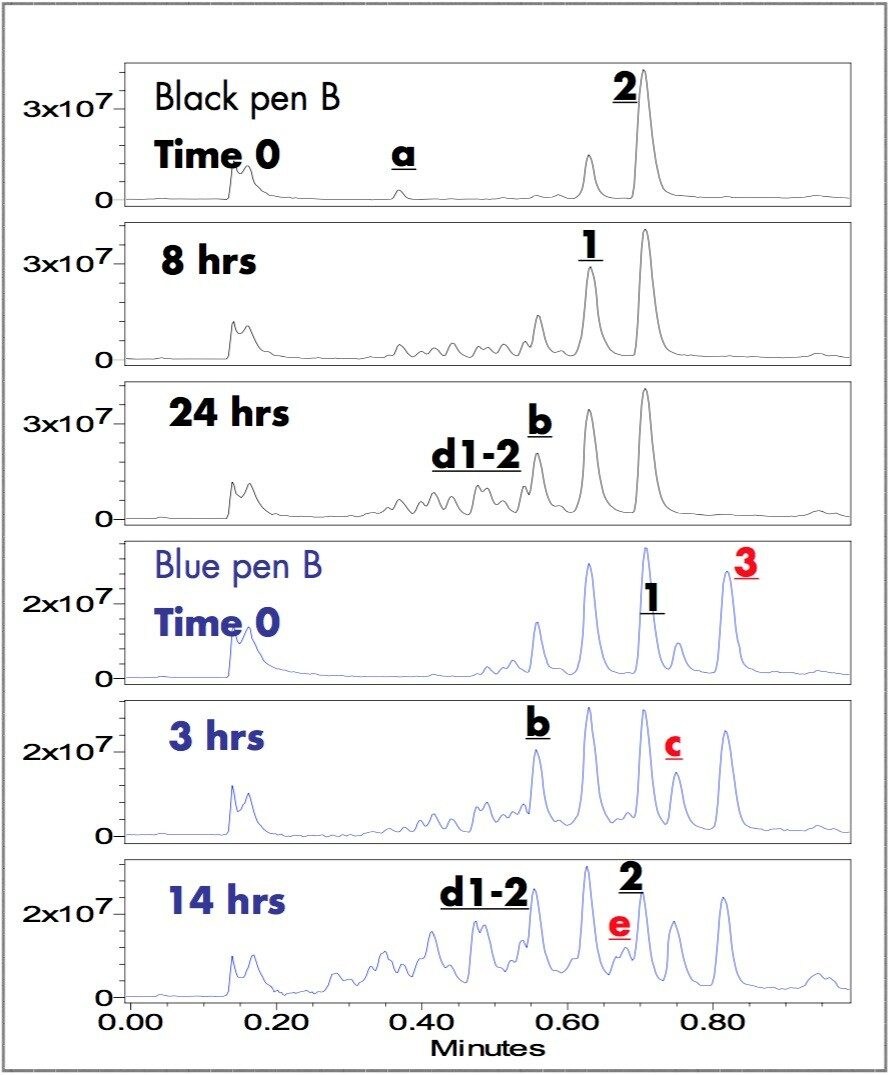
The changing peak intensity profiles of photo-degraded written samples observed using ACQUITY UPLC /UV/MS confirmed the photo-demethylation mechanism of triarylmethane dyes: 2 ⇒ 1 ⇒ b ⇒ d1-2 ⇒ ?; 3 ⇒ c ⇒ e ⇒ ?
The sensitivity, resolution and speed of the ACQUITY UPLC separation combined with UV and MS detectors for dye and ink analysis provides useful and comprehensive data in about one minute.
This application note demonstrates one application of ACQUITY UPLC in the chemical analysis market that can impact everyday life.
Sensitive, rapid analysis methods to monitor the changing profile of ink dye components extracted from written samples can aid ink manufacturers in developing more photo-stable ink formulas.
The ACQUITY UPLC combined with UV and ZQ MS Detectors provides this capability with an easy and fast method to identify structurally similar triarylmethane dyes and ink components used in ballpoint pens. The one minute separation shown here using UPLC can be applied in the analysis of impurity profiles, photo-stability and degradation mechanisms of triarylmethane dyes.
The ACQUITY UPLC/UV/ZQ MS System is a powerful tool not only to evaluate the photo-stability of ink and dye components but also to differentiate the writing from different pens, a forensic analysis application. Other applications for analyzing the stability, purity and composition of dyes using the ACQUITY UPLC System are competitive deformulation, new product development, quality control of ink dye raw materials and final products.
720001422, November 2005