This application note describes the reversed-phase separation of 9 aminoglycoside antibiotics detected using pulsed amperometric detection (PAD) on a Waters 2465 Electrochemical Detector (ECD).
Aminoglycosides are antibacterial agents used in the treatment of both animals and humans against aerobic gram-negative bacteria. More than 150 aminoglycosides have been described, all with very similar antimicrobial activity, pharmakinetic activities, and chemical structure. Liquid chromatography (LC) is the primary method of analysis for these compounds in a number of matrixes.
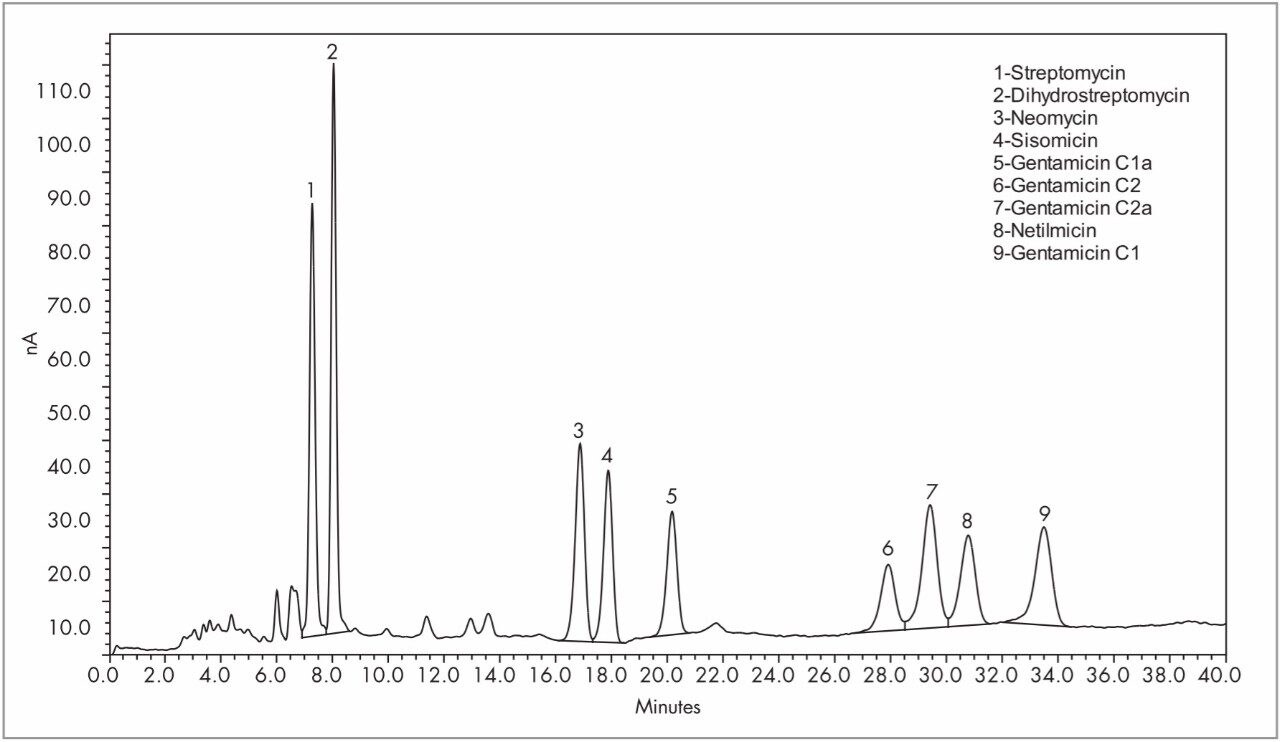
Separation of aminoglycosides is very challenging primarily due to the structurally similar nature of this compound class combined with their polar, hydrophilic character. The separation challenge is further complicated by the fact that these compounds lack a chromophore necessitating the need for an alternative detection technique. There are numerous publications that describe the analysis of these compounds using pre-column derivatization followed by using fluorescence or UV. Other detection methods, including electrochemical detection, have also been described and have been previously reviewed.1 This application note describes the reversed-phase separation of 9 aminoglycoside antibiotics (Figure 1) detected using pulsed amperometric detection (PAD) on a Waters 2465 Electrochemical Detector (ECD). Use of an ECD eliminates the need for potentially time consuming and problematic precolumn derivitization methods while providing high sensitivity, linearity and reproducibility.
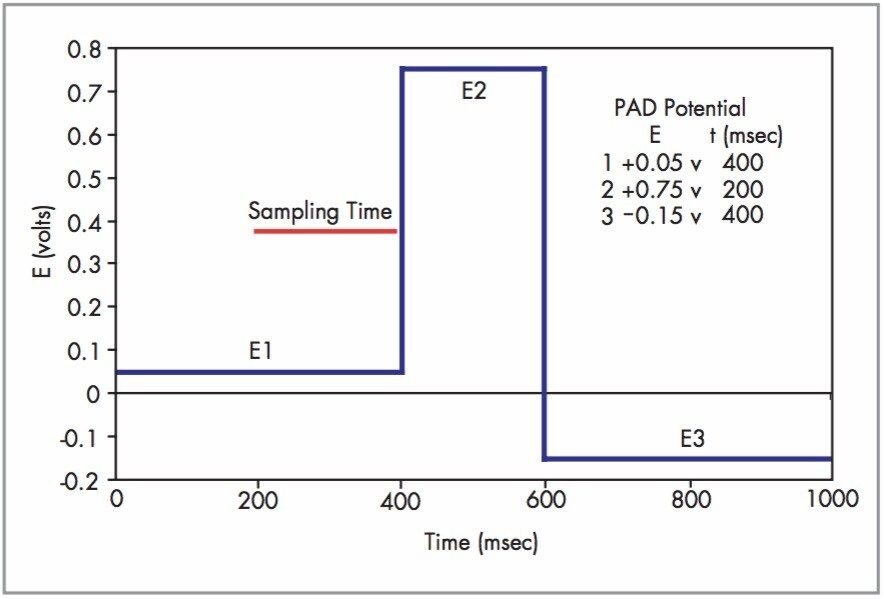
Chromatographic analysis was carried out using a Waters 2695 Separations Module combined with a 2465 Pulsed Electrochemical Detector operated in the PAD mode. The electrochemical cell consisted of a gold working electrode (3 mm diameter) combined with a hydrogen reference electrode (HyRef). Post column addition of a 20 g/L sodium hydroxide solution was made to the mobile phase using a Waters Reagent Manager operated at 0.40 mL/min through a 400 μL knitted polymeric mixing coil. The Reagent Manager is a simple, reliable, single pump used in other post column detection methods such as carbamate analysis. PAD potentials and timings are described in Figure 2. Mobile phase is similar to that used in the European Pharmacopoeia method for Netilmicin Sulfate2 and consisted of 0.02 M potassium dihydrogen phosphate (adjusted to pH 3.0 with phosphoric acid) containing 35 g/L sodium sulfate, 500 mg/L sodium octanesulfonate and 15 mL/L tetrahydrofuran. Separations were done at 1.0 mL/min on an Atlantis dC18, 5 μm, 4.6 X 250 mm. A stock solution of each aminoglycoside antibiotic (as the corresponding sulfate salt) was prepared in water. A combined standard solution was prepared by diluting stock solutions to their final concentrations with mobile phase prior to injection (5 μL, Figure 1). The final combined standard solution contained netilmicin and sisomicin at a concentration of 10.2 ng/μL, dihydrostreptomycin, neomycin, streptomycin at 6.5 ng/μL and 57.2 ng/μL of total gentamicin (a mixture of the 4 major components, C1, C1a, C2, C2a). On column amounts of the gentamicins were calculated by taking the normalized area percent of the four major gentamicin components and multiplying by the total gentamicin concentration. Increasing volumes of the mixed standard were injected (2 through 10 μL) and calibration curves for all nine compounds were generated from this data, fit to a linear regression equation, some of which are shown in Figure 3. The column, electrochemical flow cell, and mixing coil were temperature controlled at 45 °C in the 2465 detector’s integrated Faraday shielded oven. Instruments were controlled, and data collected and analyzed using Waters Empower Software.
The HyRef is a hydrogen reference electrode. It is a maintenance free, pH resistant, and highly rugged reference electrode. The electrode itself is integrated in the inlet block of the flow cell, making air bubble formation in the flow cell practically impossible. Its compatibility with high pH makes the HyRef particularly useful for any application that employs a high pH (>12). Traditional Ag/AgCl reference electrodes tend to slowly dissolve at such high pH and require regular maintenance for continued routine operation. The HyRef is stable and absolutely maintenance free under these extreme conditions and therefore was chosen over the traditional Ag/AgCl salt bridge electrode in the aminoglycoside antibiotic application. Compared to the Ag/AgCl reference electrode, signal-to-noise ratio of these two reference electrodes are comparable.
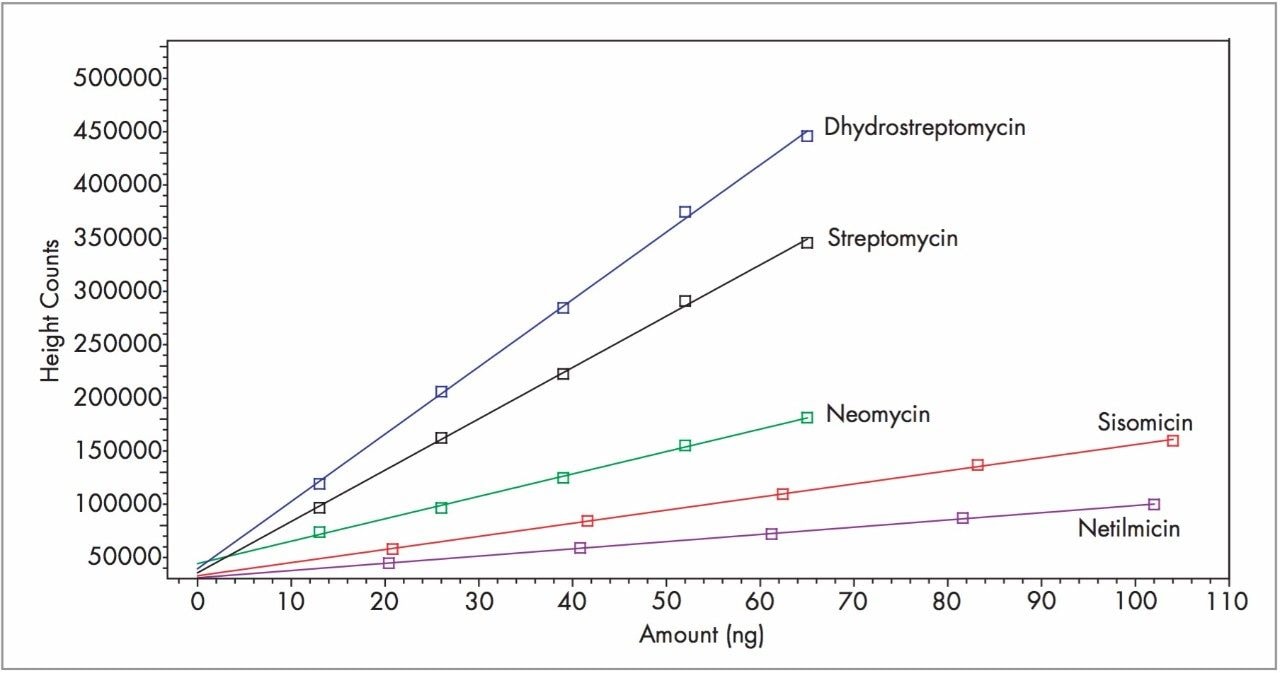
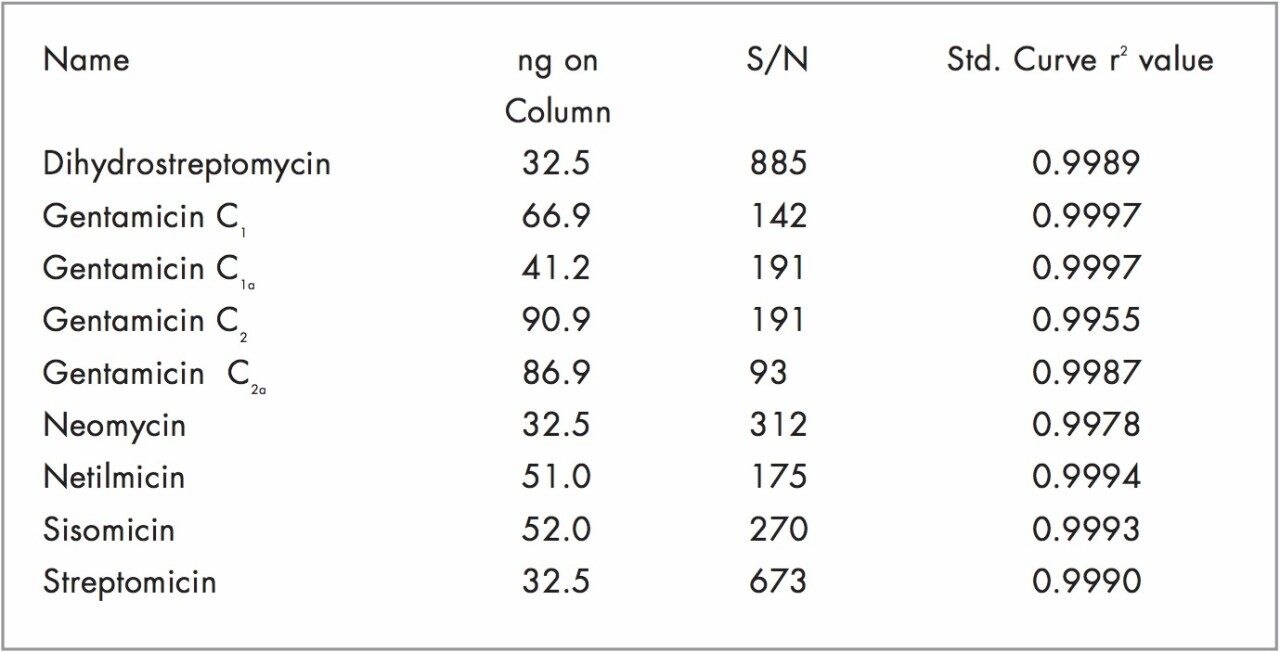
Figure 1 shows separation of the nine aminoglycoside antibiotics on the Atlantis dC18 Column. On-column amounts for Figure 1 are listed in Table 1. The Alliance System, combined with the 2465 ECD, was reproducible in both peak height counts (0.052–1.18% Relative Standard Deviation, n=6) and retention time (less than 0.015% Relative Standard Deviation, n=6) for all compounds. Figure 3 shows calibration curves for dihydrostreptomycin, streptomycin, neomycin, sisomicin, and netilmicin. Curves of the four gentamicins were similar and are not shown. Calibration curves were linear with r2 values for all compounds >0.9950 (Table 1). Excellent signal strength and low noise (<0.12 namps) produced S/N values that ranged from a high of 875 for dihydrostreptomycin to a low of 93 for gentamicin2a and are listed in Table 1. These S/N values are indicative of the highly sensitive nature of the Waters 2465 ECD.
In summary, the method presented here demonstrates that the Alliance System with a 2465 Electro Chemical Detector, under Empower control, provides highly reproducible retention times and peak response for an assay typically regarded as difficult. The Alliance System’s low noise solvent delivery, high efficiency degasser, and low carryover show it to be highly suitable for high sensitivity, demanding electrochemical applications.
Use of the 2465 in the PAD mode provides reliable, sensitive and linear response for this mixture of aminoglycoside antibiotics.
A HyRef electrode provides a much more reliable and maintenance free solution for high pH analysis that a tradition salt bridge electrode.
720000803, January 2004