For research use only. Not for use in diagnostic procedures.
In this application note, we describe a rapid and sensitive method for the simultaneous analysis of haloperidol, risperidone, and 9-hydroxyrisperidone in plasma.
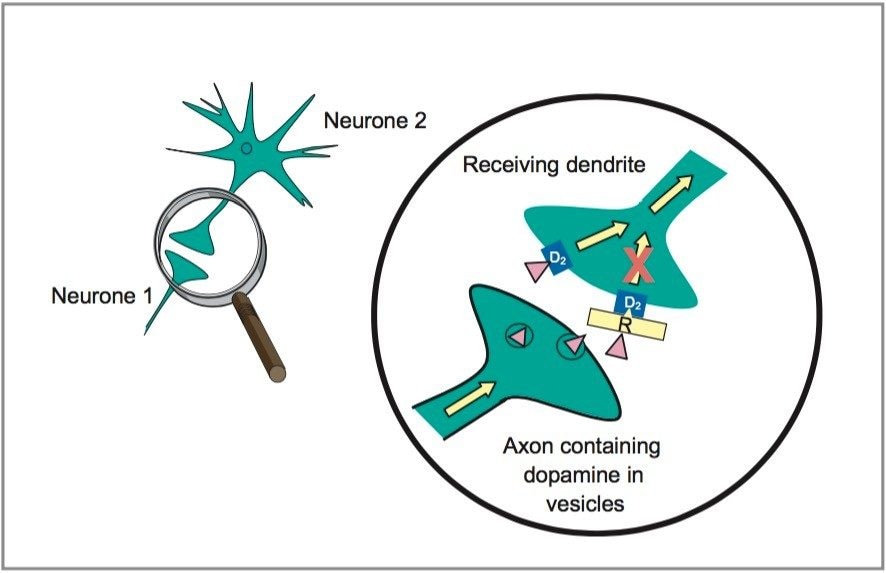
Risperidone (Risperdal) is an antipsychotic drug that is used in the management of schizophrenia. Therapeutic activity is thought to be mediated through the antagonism of both the dopamine type 2 (D2, see Figure 1) and serotonin type 2 (5-HT2) receptors of the central nervous system. Risperidone is extensively metabolized in the liver to 9-hydroxyrisperidone, which is the predominant circulating species. Since the metabolite has the same activity as the parent drug, it is important that both compounds are quantified when monitoring plasma concentrations. Although no therapeutic or toxic range has been established, concentrations are generally monitored to ensure that total drug concentrations do not exceed 200 μg/L in plasma.
Haloperidol (Haldol) is an antipsychotic tranquilizer that is used in the management of several conditions including: chronic and acute psychotic disorders, Tourette's syndrome, agitation in the elderly and also for the treatment of severe behavior problems in hyperactive children. As with risperidone, its mode of action is believed to be mediated through the D2, and to lesser extent, the 5-HT2 receptors. Typical therapeutic plasma concentrations range from 4–36 μg/L whereas levels of >50 μg/L are potentially toxic. This relatively narrow therapeutic index means that routine monitoring of patients is essential.
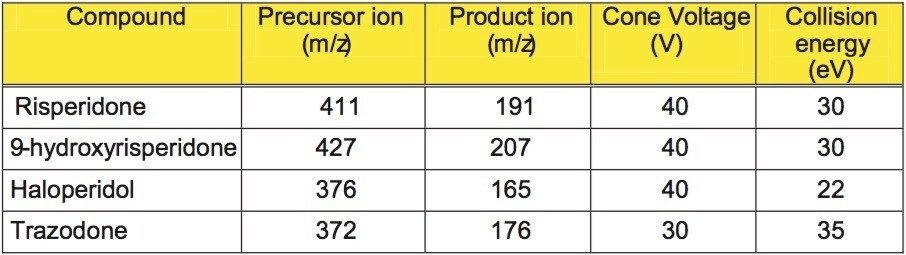
A Waters Micromass Quattro micro triple quadrupole mass spectrometer fitted with ZSpray ion interface was used for all analyses. Ionization was achieved using electrospray in the positive ionization mode (ES+). Details of the MRM conditions are given in Table 1.
Internal standard precipitant was prepared by dilution of trazodone into acetonitrile to give a final concentration of 50 μg/L. One hundred microlitres of precipitant was added to 50 μL plasma and then samples were vortex mixed briefly before centrifugation at 13,000 rpm for 10 minutes. Ten microlitres of the supernatant was analyzed by HPLC in conjunction with multiple reaction monitoring (MRM).
HPLC analyses were performed using a Waters Alliance HT 2795 Separations Module. Chromatography was achieved using a Waters Symmetry300 (2.1 x 150 mm) eluted isocratically with 2 mM ammonium acetate containing 0.1% formic acid: acetonitrile containing 0.1% formic acid (60:40) at a flow rate of 0.35 mL/min. Column temperature was maintained at 30 °C. All aspects of system operation and data acquisition were controlled using MassLynx NT 4.0 Software with automated data processing using the QuanLynx program.
We have developed a simple and rapid LC-MS/MS method that allows the simultaneous quantification of risperidone, 9-hydroxyrisperidone and haloperidol in plasma. The method, which requires only 50 μL of plasma, has a total analysis time of less than 20 minutes and comprises simple protein precipitation followed by LC/MRM analysis.
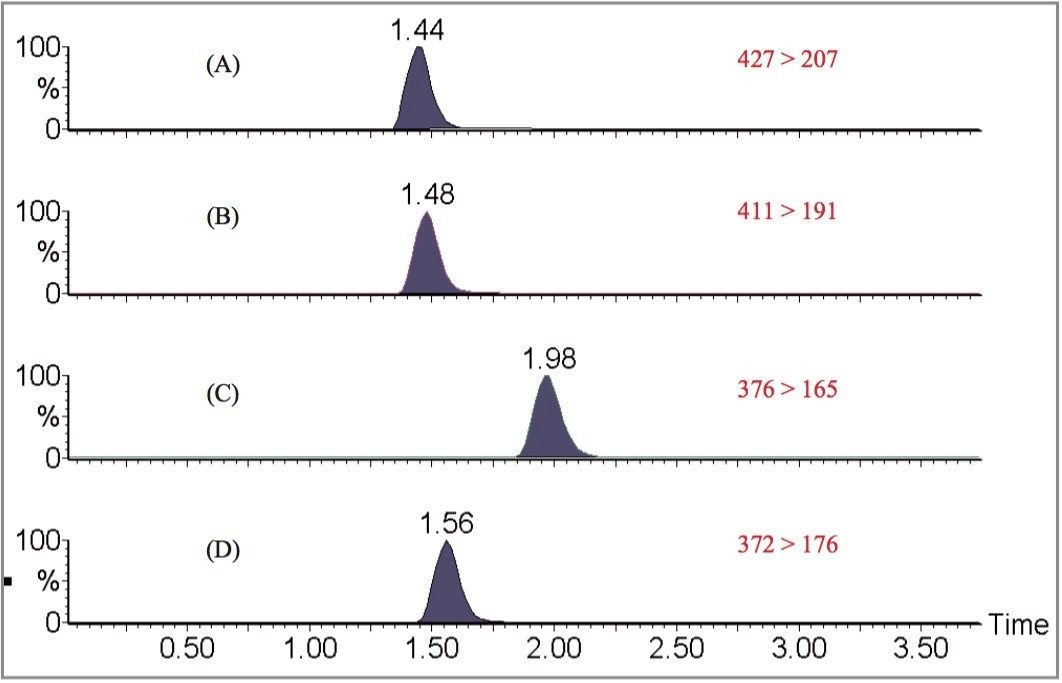
A series of calibrators (0.1–500 μg/L) were prepared by adding a mixture of the drug standards to blank plasma. The drugs were firstly isolated from the matrix, by precipitation of the proteins with acetonitrile, then analyzed using LC/MRM. Quantification was performed by integration of the area under the specific MRM chromatogram (Figure 1) and expressed in reference to the internal standard, trazodone. Linear responses were obtained for all three compounds (Correlation coefficients >0.99, 1/x weighting) over the range investigated. An example of the standard curve for risperidone is shown in Figure 2.

We describe a rapid and sensitive method for the simultaneous analysis of haloperidol, risperidone and 9-hydroxyrisperidone in plasma. The method involves a simple protein precipitation step prior to analysis using LC-MS/MS and is sufficiently sensitive to enable the quantification of these drugs in plasma taken from patients who are undergoing therapy with these particular antipsychotic agents. The wide dynamic range of the developed LC-MS/MS technique also lends itself well to the monitoring of plasma concentrations where wide inter-individual variations are often observed.
720000703, August 2003