Expanding the Antibody-Oligo Conjugate (AOC) Characterization Toolbox: Part 2- Denaturing and Non-denaturing Analysis of siRNA Payload
Samantha Ippoliti, Ying Qing Yu, Connor Brandenburg, Tara MacCulloch
Waters Corporation, United States
Note: This is the second application note in a three-part series on AOC characterization. The first application note (720009048EN) detailed the intact AOC species characterization and served as an orthogonal analysis to already published SEC-MALS results (Wyatt White Paper WP8010). A third application note outlines a novel approach to conjugation site determination (720009079EN).
Published on October 24, 2025
Abstract
Short interfering RNA (siRNA) is an important class of molecules under active development. Recently, researchers have been investigating the benefits of conjugating siRNA to monoclonal antibodies (mAbs) via a chemical linker, creating an antibody-oligonucleotide conjugate (AOC). To ensure the best possible AOC drug product, siRNA and linker-siRNA intermediates must be fully characterized prior to the conjugation step. Typically, synthetic oligonucleotides are analyzed via ion-pairing reversed phase (IPRP) chromatography coupled to mass spectrometry (MS). IPRP chromatography, though effective for oligonucleotide analysis, has some drawbacks; most notably, the ion-pairing reagents tend to have toxic properties and are difficult to eliminate from UPLC™ instruments. Hydrophilic interaction chromatography (HILIC)-MS has recently emerged as a promising alternative for both denatured and native siRNA characterization.1 Compared to IPRP, HILIC-MS employs less toxic reagents and facilitates a smoother transition between oligonucleotide and protein analysis types on a single LC-MS instrument. In this study, both HILIC-MS and IPRP-MS approaches were evaluated for effectiveness of siRNA analysis using the BioAccord™ LC-MS System.
Benefits
- Orthogonal denaturing and non-denaturing techniques were applied for the characterization of the siRNA building blocks of AOC therapeutics
- Intact AOC and siRNA analysis are capable of being performed on the same benchtop time-of-flight (ToF) LC-MS system operated under compliance-ready waters_connect™ Informatics Platform
- Robust, reliable MaxPeak™ Premier Column chemistries and MS-grade IonHance™ Mobile Phase Concentrates facilitated easy method setup and transitions between assays
Introduction
Oligonucleotides continue to grow as an emerging class of biotherapeutic molecules being studied for a range of indications. For example, small interfering ribonucleic acid (siRNA), one type of oligonucleotide, is capable of regulating protein expression in vivo. Thus, they are an effective modality for a wide range of disease treatments.1 When an oligonucleotide is conjugated to a monoclonal antibody (mAb), the antibody serves as a targeting mechanism, directing the therapeutic oligonucleotide to a specific cell type. This targeted delivery enables the oligonucleotide to exert its therapeutic effect precisely where it is needed and reduce undesirable off-site activities. In an AOC, the oligonucleotide is chemically conjugated to the mAb via a cleavable linker to create the efficacious molecule (Figure 1).
siRNA molecules are comprised of ~20–25 base pair oligo strands which are present as a non-covalent stable duplex.2 LC-MS analysis of oligonucleotides via IPRP chromatography coupled to MS is typically a denaturing technique.3–4 In the case of siRNA, the two strands will dissociate under these conditions, and the resulting sense and antisense strands can be analyzed individually for molecular weight confirmation. Recent studies with HILIC-MS of siRNA have shown that it is possible to use the same LC-MS conditions, with only a difference in column temperature, to achieve both denatured and non-denatured siRNA species for analysis.1 Though it was found to be a less sensitive assay, HILIC-MS is an attractive alternative to IPRP-MS because it does not use traditional ion-pairing reagents, the use of which require careful consideration. Ion pairing reagents tend to be difficult to eliminate from LC pumps and flow paths. Therefore, many labs dedicate certain LC systems as “IPRP only” systems to avoid the need for stringent cleaning procedure before switching to a different chromatographic mode, and avoid the resulting system downtime. In addition, the reagents for IPRP are more toxic and less environmentally friendly, so reducing their prevalence in analytical labs is beneficial. In this study, both approaches have been investigated for the characterization of the free and linker-functionalized siRNA components that would typically be employed in support of an AOC development campaign. The same BioAccord LC-MS System (Figure 1) that was used in the first application note of the series (720009048EN) for the AOC characterization was also used in this analysis.

Experimental
Sample Preparation (siRNA analysis)
Samples of MCC (maleimidomethyl cyclohexane-1-carboxylate) linker functionalized siCOL1a1 siRNA (MCC-siCOL1a1) and free siCOL1a1 were diluted to 5 µM in HILIC-MS mobile phase A (50 mM Ammonium Acetate, pH 6.8), or IPRP-MS mobile phase A (80 mM HFIP, 7 mM TEA in water, pH 9). Samples were generously supplied by Takeda Pharmaceuticals.
Method Conditions
LC Conditions
|
LC system (all): |
ACQUITY™ Premier System |
|
Detection (all): |
ACQUITY UPLC TUV Detector (260 nm) |
|
Sample temperature (all): |
6 °C |
|
Column(s): |
HILIC-MS: ACQUITY Premier Glycoprotein BEH™ Amide Column, 300Å, 1.7 µm, 2.1 x 50 mm (p/n:186009547) IPRP-MS: ACQUITY Premier Oligonucleotide BEH C18 Column, 130Å, 1.7 µm, 2.1 x 50 mm (p/n: 186009484) |
|
Column temperature: |
HILIC-MS: 35 °C (non-denaturing) and 90 °C (denaturing) IPRP-MS: 80 °C (denaturing) |
|
Injection volume: |
HILIC-MS & IPRP-MS: 10 µL 5 µM siCOL1a1 and MCC-siCOL1a1 samples |
|
Flow rate: |
HILIC-MS: 0.4 mL/min IPRP-MS: 0.3 mL/min |
|
Mobile phases: HILIC-MS |
MPA: 50 mM Ammonium Acetate, pH 6.8 (created from IonHance Ammonium Acetate Concentrate, p/n: 186009705) MPB: 100% Acetonitrile |
|
Mobile phases: IPRP-MS |
MPA: 80 mM HFIP (hexafluoro-2-propanol), 7 mM TEA (triethylamine) in water, pH 9 MPB: 40 mM HFIP, 3.5 mM TEA in 50% Methanol (e.g. 50% MPA + 50% Methanol) |
Gradient Table: HILIC-MS
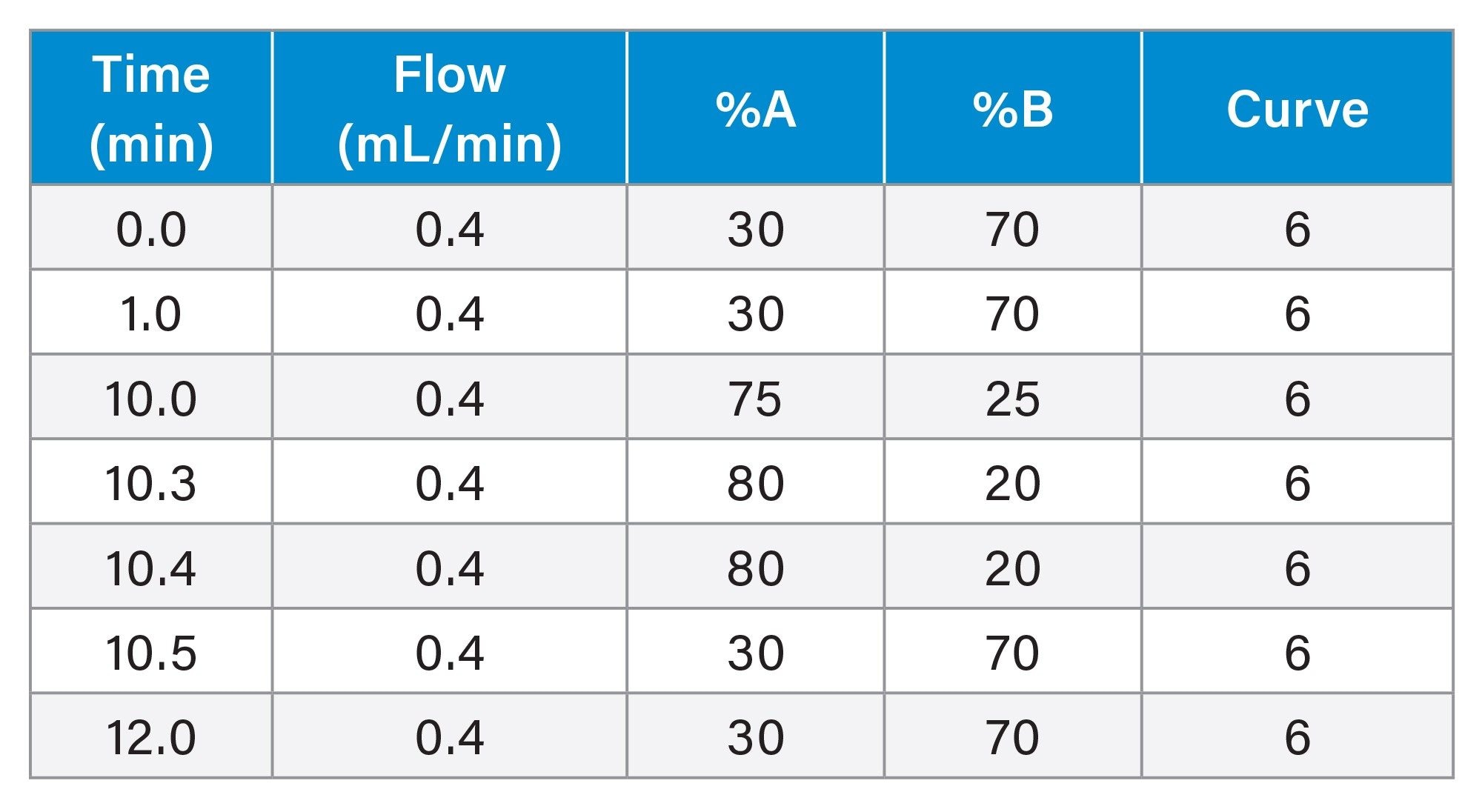
Gradient Table: IPRP-MS
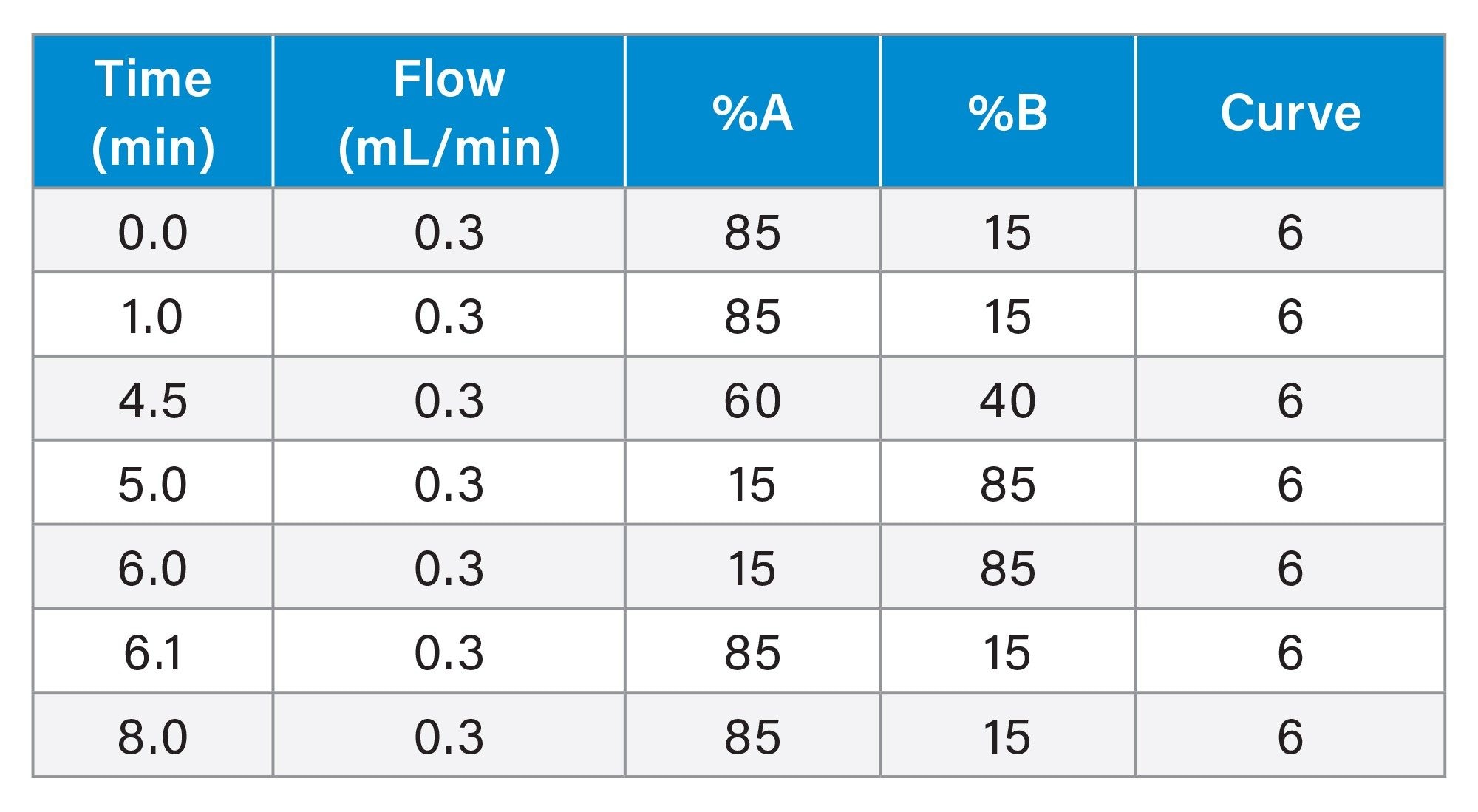
MS Conditions
|
MS system: |
ACQUITY RDa™ Mass Detector |
|
Ionization mode: |
ESI Negative, Full Scan with Fragmentation |
|
Acquisition range: |
400–7000 m/z (High Mass) |
|
Scan rate: |
2 Hz |
|
Capillary voltage: |
0.80 kV |
|
Cone voltage: |
40 V |
|
Fragmentation cone voltage: |
40–200 V |
|
Desolvation temperature: |
550 °C |
|
Intelligent data capture: |
On |
|
Lockmass: |
Standard |
Data Management
All data was acquired through the UNIFI™ Application (v 3.1.0.16) and processed with INTACT Mass Application (v 1.9) within the waters_connect Informatics Platform (v 3.1). IPRP-MS data was also processed with CONFIRM Sequence App (v 2.0) to confirm the location of the linker incorporated on the 3’ end of the sense strand.
Results and Discussion
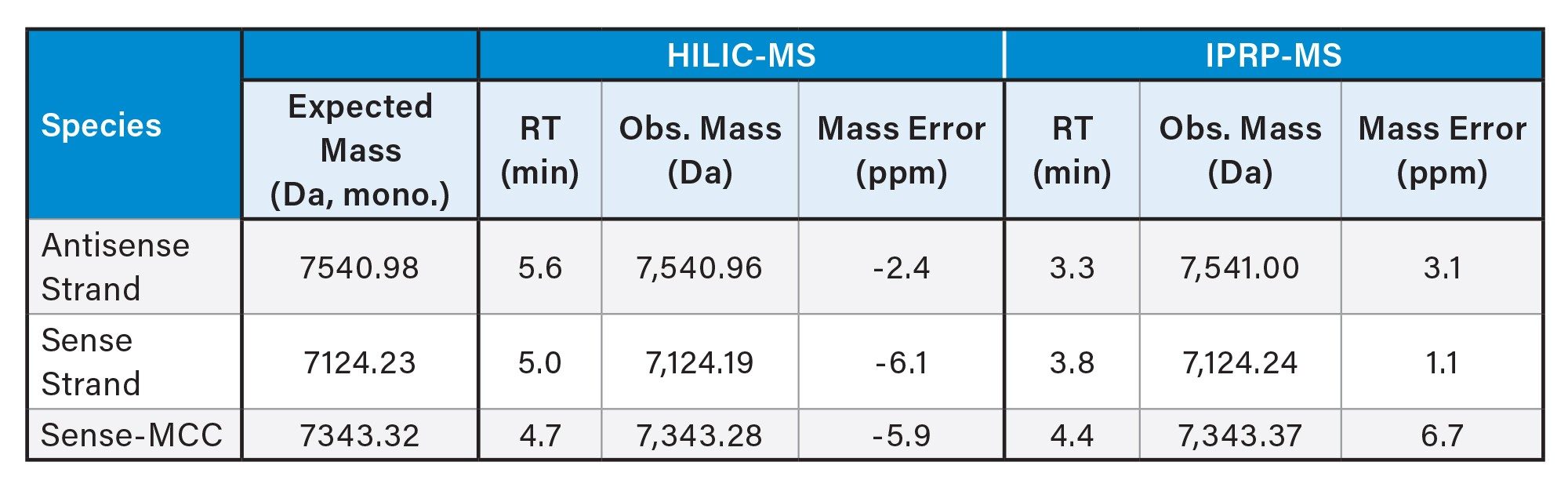
This application note outlines the characterization of the free siCOL1a1 siRNA and siCOL1a1-MCC (siRNA + linker) that was used to create the AOC samples detailed in the first application note of this series and an orthogonal analysis with SEC-MALS study.5 HILIC-MS (denaturing & non-denaturing) and IPRP-MS (denaturing) were evaluated for the characterization of these siRNA species. The expected molecular weights (monoisotopic masses) for the free siCOL1a1 and siCOL1a1-MCC are 14,665.21 Da and 14,884.30 Da, respectively. (The expected delta mass of +219.09 Da is derived from the mass of the MCC linker (236.17 Da) after the reaction of the N-hydroxysuccinimide (NHS) ester group of the linker to the primary amine on the 3’ end of the siRNA sense strand.)
Denaturing vs Non-denaturing HILIC-MS
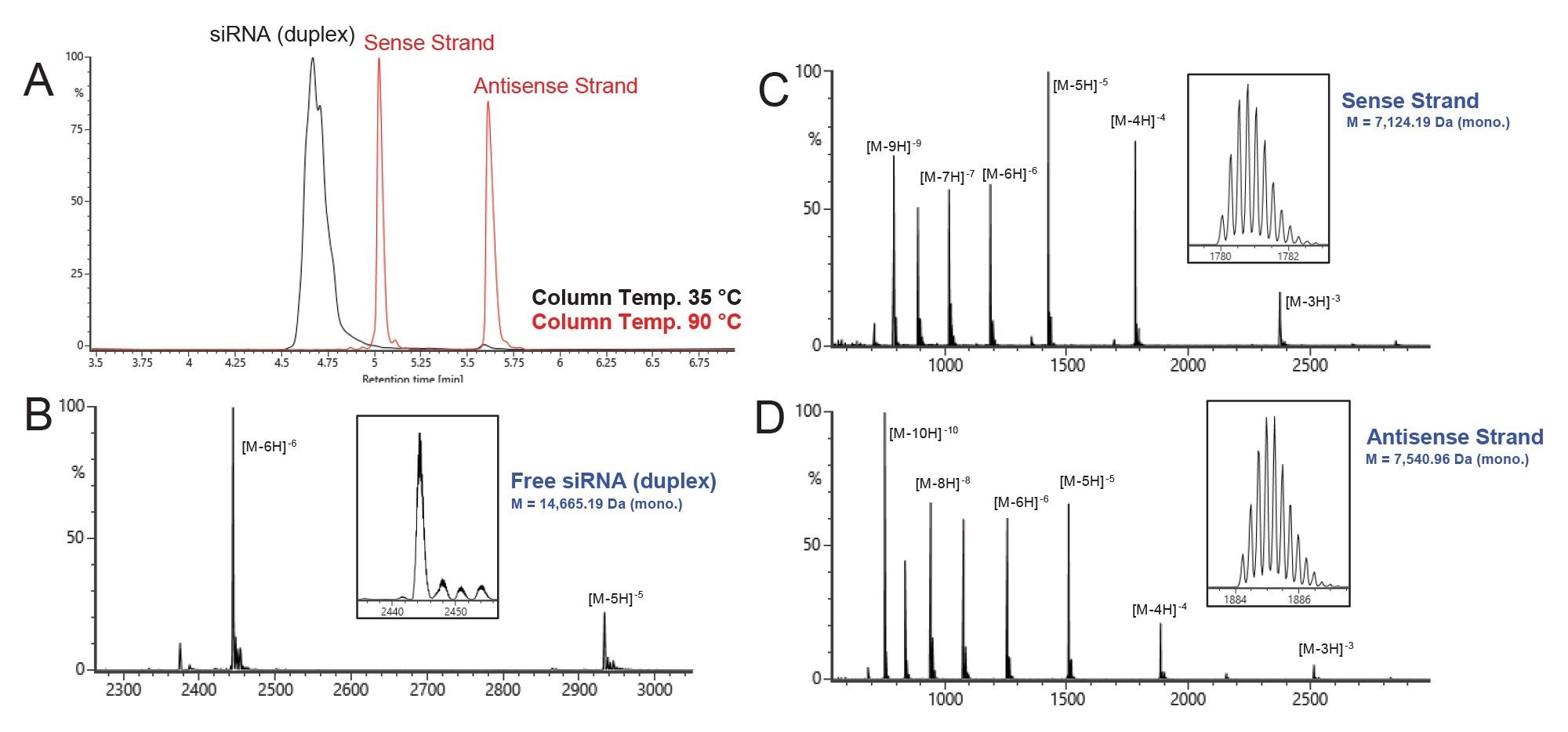
Denaturing and non-denaturing HILIC-MS were first tested using conditions based on previous analysis of siRNA duplexes.1 One can use the same LC conditions and only change the column temperature to induce on-column dissociation of the sense and antisense strands for analysis. Experience with this set of samples showed the original column temperature of 60 °C was not strong enough to dissociate the siRNA duplex. Literature research revealed that siRNA may exhibit different melting temperatures that are affected by various buffer conditions.6 Under typical HILIC conditions, the melting temperature was found to be ~80 °C. The analysis was then repeated with a column temperature of 90 °C for the denaturing analysis, yielding a more effective denaturation and separation of the siRNA strands. Figure 2A shows the UV overlay of chromatograms for the free siCOL1a1 sample, comparing the non-denaturing (35 °C, black trace) and denaturing (90 °C, red trace) HILIC conditions. Figures 2B, 2C, and 2D show the combined raw summed spectra from each of the peaks (2B = non-denaturing analysis, RT 4.6 min; 2C & 2D = denaturing analysis, RT 5.1 & 5.6, respectively). Each panel also contains a zoomed view of the largest charge state and the deconvoluted mass for each species, reported as monoisotopic mass.
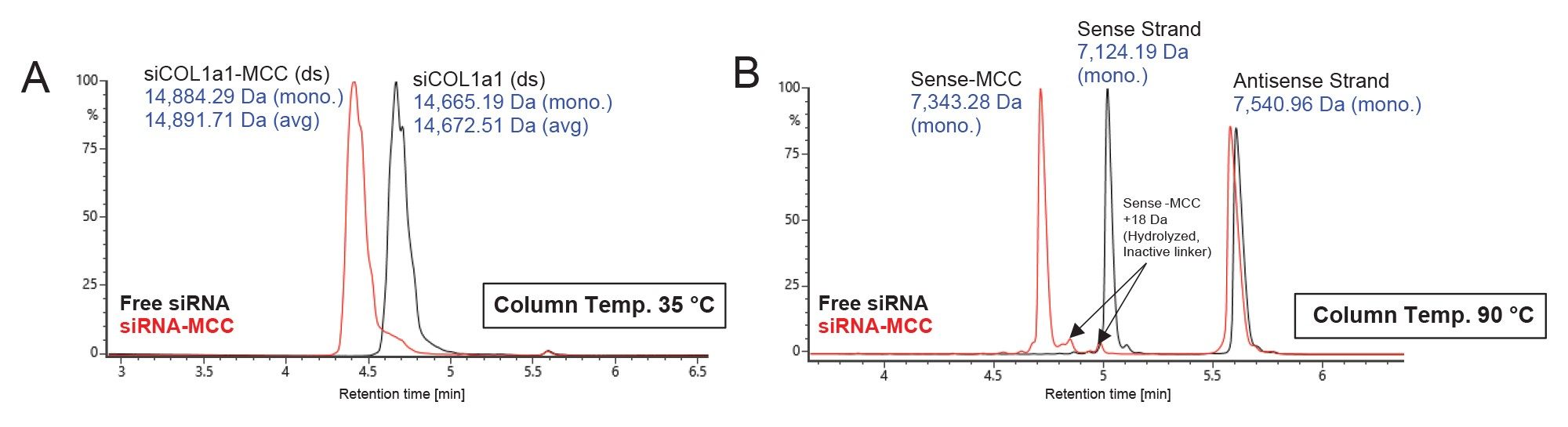
Analysis of siCOL1a1 vs siCOL1a1-MCC by HILIC-MS
Figure 3 shows UV overlays comparing free siCOL1a1 (black traces) and siCOL1a1-MCC (red traces) under non-denaturing (Fig 3A) and denaturing (Fig 3B) HILIC conditions. The free siCOL1a1 generated a single duplex peak in non-denaturing mode and two expected single-strand peaks in denaturing mode. In the siCOL1a1-MCC sample, LC-MS data demonstrated that the conjugation occurred selectively on the sense strand. The antisense strand (mass 7,540.96 Da, RT 5.6 min) was unchanged, whereas the UV & MS signal for the sense strand (mass 7,124.19 Da, RT 5.0) decreased and a new peak corresponding to Sense-MCC appeared (mass 7,343.28 Da, RT 4.7 min). An additional impurity corresponding to a hydrolyzed Sense-MCC species (+18 Da) was detected. Monitoring this impurity is critical, as unreactive hydrolyzed linker reduces the effective molar ratio of reactive oligo-linker to antibody in subsequent AOC reactions. Adjustment of the linker equivalents or further purification may be required to achieve the intended stoichiometry.
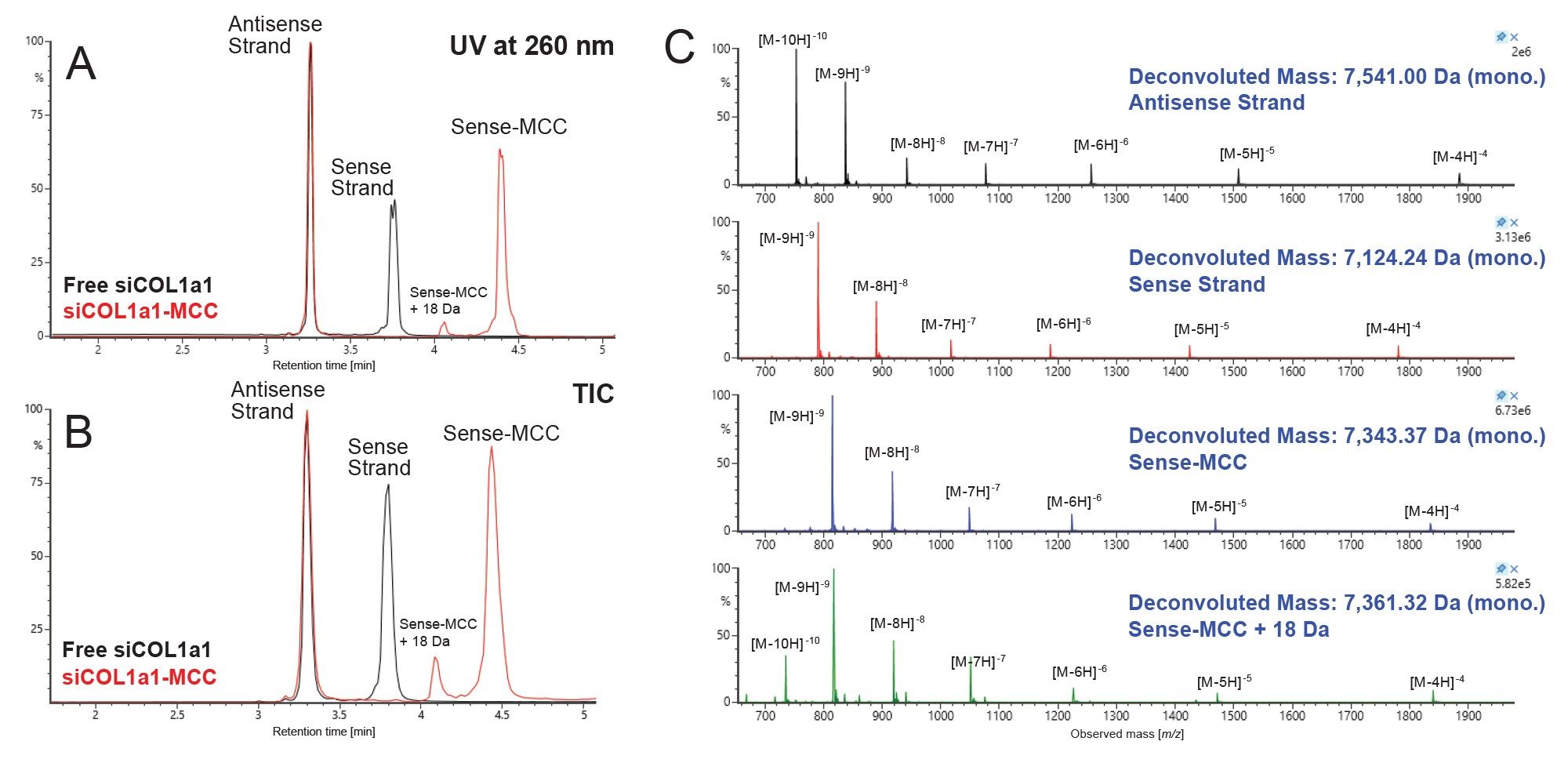
Orthogonal IPRP-MS Analysis
To complement HILIC-MS analysis, a widely established technique for oligonucleotide analysis, IPRP-MS, was also applied. Figure 4 shows the UV overlay (Figure 4A) and total ion chromatogram (TIC) (Figure 4B) for the IPRP-MS analysis of the free siCOL1a1 (black) and siCOL1a1-MCC (red) injections. Figure 4C shows the combined raw MS spectra for each main peak, in order of elution. Each of the same species observed in denaturing HILIC-MS is also observed using IPRP-MS, though in reverse elution order, as expected based on selectivity differences. Consistent with the denaturing HILIC-MS results, the antisense strand remains constant between samples, and the MCC linker is observable on only the sense strand.
Data Processing with INTACT Mass App
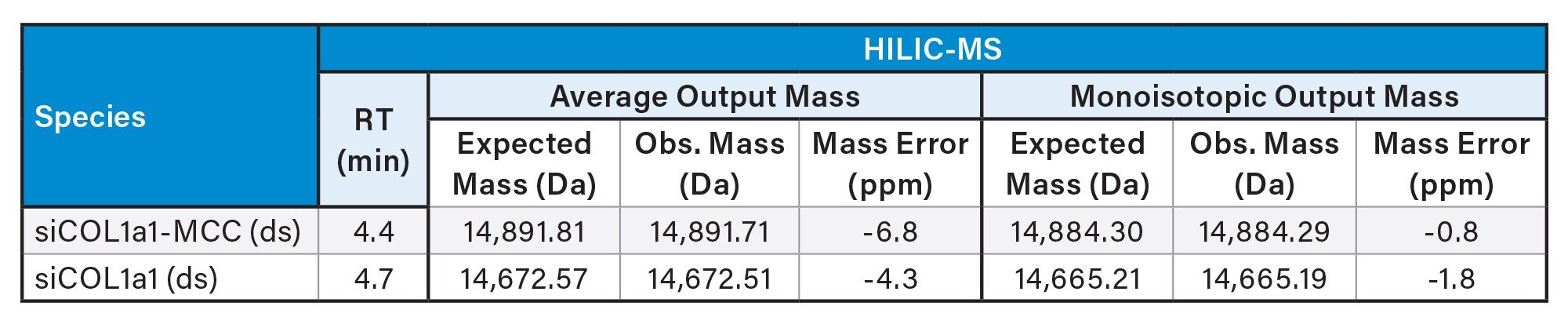
All HILIC-MS and IPRP-MS datasets were processed with the INTACT Mass App in the waters_connect Informatics Platform. For strands under 15 kDa, BayesSpray deconvolution with monoisotopic mass output was applied. Both sense and antisense strands (7-7.5 kDa) were correctly deconvoluted, and variable modification searching (including MCC linker) enabled all species to be matched. Mass accuracies were consistently < 10 ppm for denatured samples (Table 1). For the non-denatured duplex, with an expected mass nearing the 15 kDa threshold, the data was processed using both monoisotopic and average-mass output reporting. Each approach provided accurate assignments within 15 ppm (Table 2). A new functionality available in version 1.9 of INTACT Mass App allows the user to enter chemical formula to be used for the deconvolution, which even further improved the mass accuracy for monoisotopic-mass output for these samples (< 2 ppm).
Linker Localization
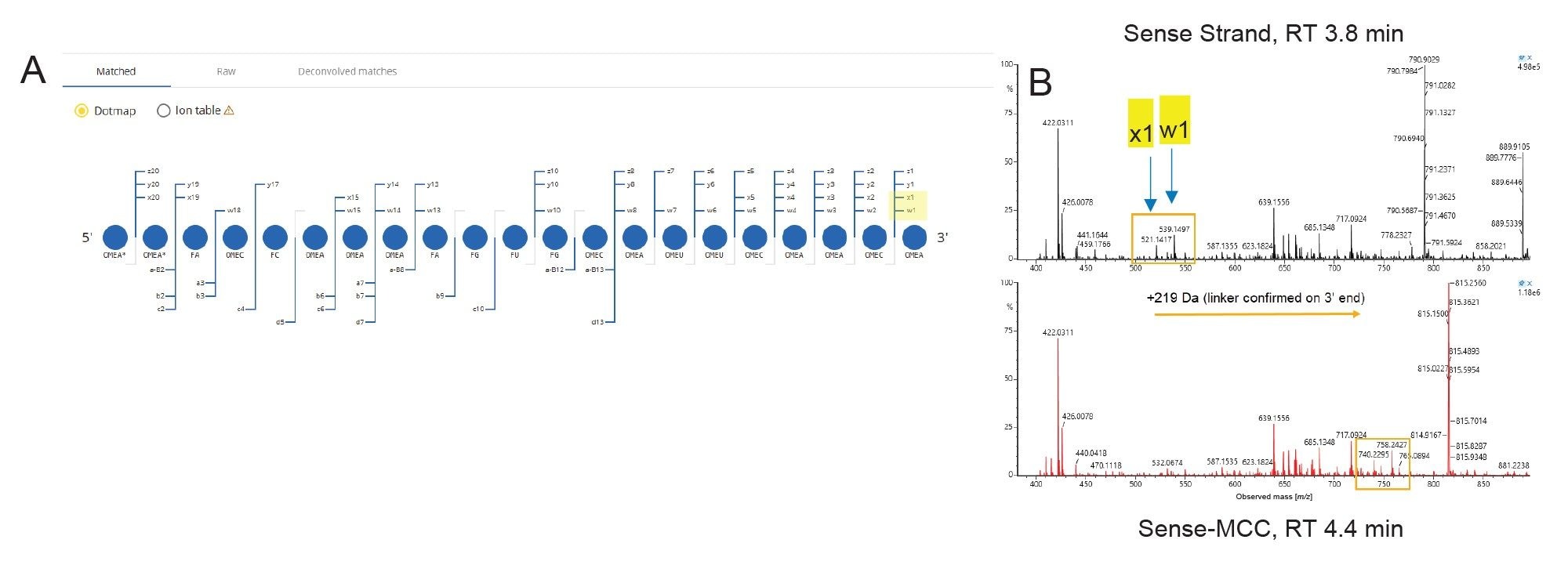
Lastly, to confirm that the MCC linker was incorporated into the 3’ end of the sense strand as expected, the fragmentation data from the IPRP-MS was processed with the CONFIRM Sequence App (also embedded in the waters_connect Platform) to match the fragment ions with expected CID fragmentation products. The Dotmap plot of the sense strand (Figure 5A) shows the fragments which were matched with the theoretical fragments of the free siCOL1a1 sense strand—90% sequence coverage was observed. For the antisense strand, 78% sequence coverage was observed (data not shown). Figure 5B shows the combined raw spectra of the high energy channel from the peak corresponding to the sense strand at 3.8 min (black) compared to raw spectra of the peak corresponding to the sense-MCC linker at 4.4 min (red). Fragment ions x1 and w1 were found with the mass shift of +219 Da corresponding to the MCC linker, confirming the linker location on the 3’ end of the sense strand.
Conclusion
Denaturing and non-denaturing HILIC-MS, together with IPRP-MS, enabled comprehensive characterization of free siCOL1a1 siRNA and the siCOL1a1-MCC conjugate. HILIC-MS and IPRP-MS under denaturing separation conditions provided concurrent and accurate results for the denatured siRNA strands and linker-reacted species present. HILIC-MS under non-denaturing separation conditions provides confirmation of the correctly formed intact siRNA species. The ability to carry out these component level studies on the same platform that enabled analysis of the assembled AOC molecules provides for more efficient use of lab resources for AOC studies. In addition, these results highlight the value of an integrated LC-MS informatics platform in accelerating drug development workflows. By delivering confident molecular characterization and impurity tracking, this approach reduces risk in AOC manufacturing, supports regulatory readiness, and differentiates the BioAccord System as a highly versatile analytical tool for emerging antibody-oligonucleotide conjugates.
References
- Finny A, Johnston T, Sauner B, Addepalli B, Lauber MA. Reproducible Hydrophilic Interaction Chromatography for Denaturing and Non-Denaturing Analyses of Oligonucleotides Using GTxResolve Premier BEH Amide Columns. Waters Application Note 720008456. August 2024.
- Yogendrarajah P, Marina IS, Verluyten W, Dejaegere E, Napoletano L, Boon JP, Hellings M, Gilar M. Analysis of siRNA with Denaturing and Non-Denaturing Ion-Pair Reversed-Phase Liquid Chromatography Methods. LCGC North America. 41:2 (2023).
- Doneanu C, Boyce P, Shion H, Fredette J, Berger SJ, Gastall H, Yu YQ. LC-MS Analysis of siRNA, Single Guide RNA and Impurities Using the BioAccord™ system with ACQUITY™ Premier and New Automated INTACT Mass Application. Waters Application Note 720007546. April 2022.
- Shion H, Doneanu C, Ha E, Yu YQ, Chen W. Analysis of Antibody siRNA Conjugate Using BioAccord System. Waters Application Note 720007212. March 2021.
- Brandenburg C, Liu H, Kenrick S. WP8010: Determination of Multiple Quality Attributes of Antibody-Oligonucleotide Conjugate with SEC-MALS and AEX-MALS. Waters-Wyatt White Paper. 2024.
- Gilar M, Redstone S, Gomes A. Impact of mobile and stationary phases on siRNA duplex stability in liquid chromatography. J Chrom A. 1733, 465285 (2024). http://doi.org/10.1016/j.chroma.2024.465285
BioAccord, waters_connect, ACQUITY, MaxPeak, IonHance, BEH, RDa, and UNIFI are trademarks of Waters Technologies Corporation.
Acknowledgement
Special thanks to Martin Gilar (Waters Corporation) for technical guidance in the field of oligonucleotide separations.
720009066, September 2025