Breaking Down RNA Stability: Accelerated Aging of Reference Standards with ASAPprime® Modeling
Kristina Flavier1, Chetan Shende1, Justin McCabe1, Christian Reidy2, Makda Araya2, Balasubrahmanyam Addepalli2, Koley Hall3
1 FreeThink Technologies, Inc, Branford CT, USA
2 Waters Corporation, Milford MA, USA
3 Waters Corporation, Golden CO, USA
Published on August 13th, 2025
Abstract
Access to high-quality RNA reference materials is essential for method qualification and system suitability assessments in both development and quality control (QC) environments. Waters™ offers a selection of ready-to-analyze lyophilized oligonucleotide and nucleic acid standards with diverse molecular compositions and sizes to support these applications. These standards enable rapid evaluation of method performance, column integrity, and system benchmarking across a range of chromatographic techniques, including size-exclusion chromatography (SEC), ion-pair reversed-phase (IP-RP), hydrophilic interaction liquid chromatography (HILIC), and anion-exchange (AEX). A clear understanding of the stability characteristics of these reference materials is critical to ensure reliable system performance and method assessment following reconstitution. FreeThink Technologies offers a methodology and software (ASAPprime®) that enables rapid determination of shelf life using statistically designed exposure of samples to a range of temperature and relative humidity conditions. The Accelerated Stability Assessment Program (ASAP) process was applied to test RNA reference materials and successfully determine long-term shelf life based on three weeks of predictive stability data.
Benefits
- Evaluate the shelf life and stability of Waters Lipid-Conjugated Antisense Oligonucleotide (ASO) LC-MS and siRNA LC-MS Reference Standards
- Use HILIC chromatography to assess impurity profiles in oligonucleotide samples
- Apply the ASAP in conjunction with ASAPprime® software to model long-term stability and support optimal packaging and storage recommendations
Introduction
Analytical standards and reagents play a vital role in ensuring accuracy, reproducibility, and regulatory compliance in oligonucleotide analysis. As oligonucleotide-based therapeutics continue to grow in complexity, access to high-quality reference materials is increasingly important for streamlining analytical workflows, verifying system suitability, and maintaining method robustness over time.
Oligonucleotides are sensitive to environmental factors such as temperature, humidity, oxygen, and contaminants, all of which can contribute to degradation. Lyophilization (freeze-drying) is a widely used technique to improve the stability and shelf life of oligonucleotide standards by converting them from solution into a dry, solid form. When performed in a sealed container, lyophilization removes the majority of water, thereby reducing hydrolytic degradation, molecular mobility, and nuclease activity. Reducing these degradation pathways enhances long-term stability, particularly when materials are stored under frozen conditions (-20 °C) due to the slowdown in degradation rates with decreasing temperature. To help mitigate the risk of degradation, Waters Lipid-Conjugated ASO and siRNA LC-MS Reference Standards are lyophilized in capped tubes, sealed in foil pouches, and stored at -20 °C to ensure optimal stability.
The ASAP incorporated into the commercial software packaging ASAPprime® determines long-term stability in a much shorter timeframe as compared to the traditional one to five years typically used for real-time testing. Samples are stressed at a range of temperature and relative humidity conditions outside of packaging for up to one month, and the isoconversion time, or time to reach the failure point, is determined at each condition.1 The isoconversion times at each stress condition are used to quantitatively calculate the temperature and moisture sensitivities of degradation. Based on these parameters, the ASAP model is then used to predict shelf life at the intended storage conditions and in different packaging configurations. The ASAP approach is most commonly used for pharmaceuticals but can also be applied to any type of product change that is predictively accelerated by temperature, including oligonucleotide degradation.2
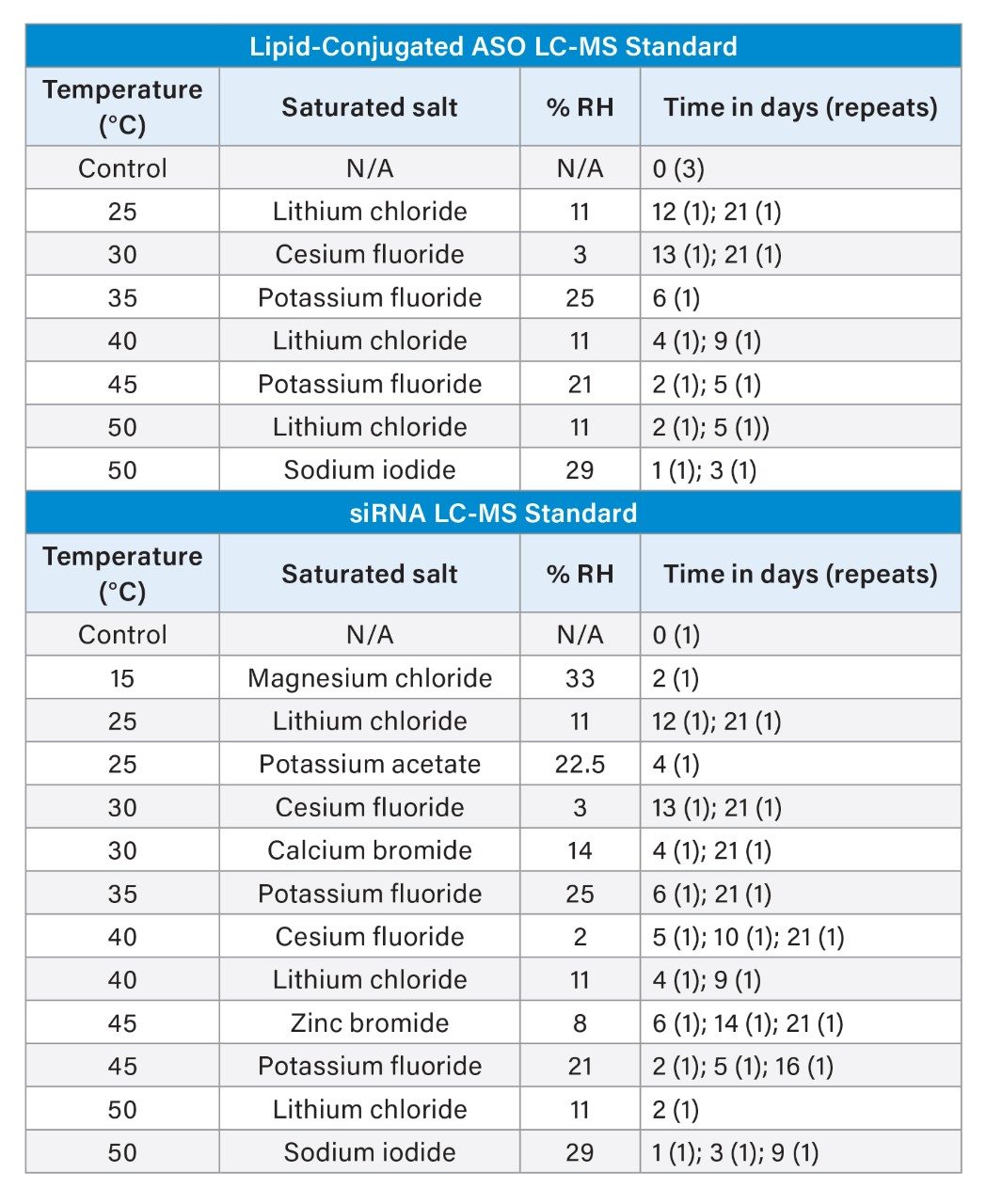
FreeThink Technologies conducted an ASAP study to model the stability of Waters Lipid-Conjugated ASO LC-MS and siRNA LC-MS Standards. In this study, the standards were removed from their packaging and exposed to a range of controlled temperatures and relative humidities designed to accelerate the aging process. The standards have a defined purity specification of >90%, which was used as the basis for determining acceptable levels of change for each standard (8% and 10% for the Lipid-Conjugated ASO LC-MS and siRNA LC-MS Standards, respectively). Purity loss was monitored using HILIC methods, and the resulting data were used to extrapolate stability under frozen (-20 °C) and refrigerated (4 °C) storage conditions, supporting a projected 2-year shelf life.
Experimental
Experimental Table
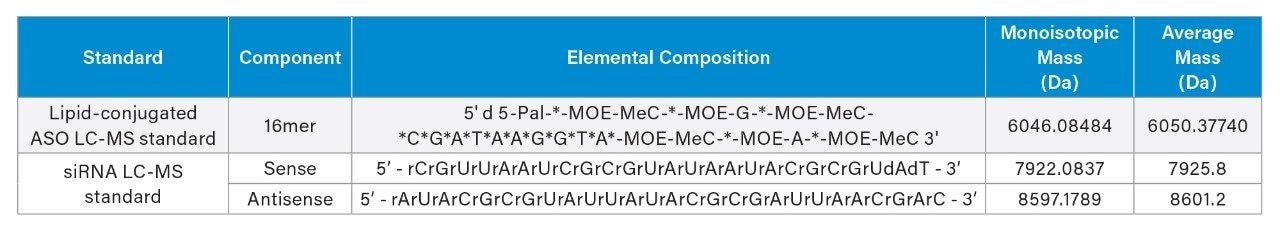
Vials of the Lipid-Conjugate ASO LC-MS Standard (p/n: 186010747) and siRNA LC-MS Standard (p/n: 186010598) were provided to FreeThink Technologies by Waters Corporation. The Lipid-Conjugated ASO LC-MS Standard contains 5 nmol quantity of a 16 residue gapmer antisense oligonucleotide (ASO LC-MS) with a fully phosphorothioated backbone, a 5’ palmitate modification, and methoxy ethyl modified termini. The siRNA LC-MS Standard contains 1 nmol quantity of a mixture of annealed 25-mer and 27-mer RNA strands.
For each ASAP condition, one vial of siRNA LC-MS Standard and one vial of the Lipid-Conjugated ASO LC-MS Standard were placed without caps (open) into a canning jar with a third vial containing the appropriate saturated salt to control the humidity inside the jar (Table 1). The canning jars were sealed and placed into the appropriate ovens in a staggered fashion such that all samples were removed at the end of the study. The siRNA LC-MS Standard demonstrated a very high sensitivity to humidity, requiring the use of milder temperature and humidity conditions than for the Lipid-Conjugated ASO LC-MS Standard to generate data close to the failure point. Control samples were stored refrigerated and unopened for the duration of the study before being prepared for ultra-performance liquid chromatography (UPLC™) analysis simultaneously with stressed samples.
Stressed Lipid-Conjugated ASO LC-MS Standard (15 nmol/mL):
At each timepoint following stressing, the standard was reconstituted by adding 50 µL of 50:50 acetone:ACN and gently vortexing to ensure complete dissolution. A 15 µL aliquot of the resulting solution was then transferred to a vial containing 985 µL of 50:50 acetone:ACN and mixed thoroughly. The sample was placed in the autosampler for analysis. Unaged control samples were prepared following the same procedure for direct comparison.
Stressed siRNA LC-MS Standard (5 nmol/mL):
At each timepoint following stressing, the standard was reconstituted by adding 200 µL of 50:50 ACN:50 mM ammonium acetate and gently vortexing to ensure complete dissolution. The sample was placed in the autosampler for analysis. Unaged control samples were prepared following the same procedure for direct comparison.
HILIC Conditions
|
LC system: |
ACQUITY™ UPLC H-Class System with Flow-Through Needle (FTN) |
|
Column: |
GTxResolve™ Premier BEH™ Amide, 300 Å, 1.7 µm, 2.1 X 100 mm Column (p/n: 186011250) |
|
Sample temperature.: |
5 °C |
|
Flow rate: |
0.3 mL/min |
|
Needle wash: |
50:50 ACN:water (v:v) |
Detector Conditions
|
Detection: |
260 nm |
|
TUV sampling rate: |
5 Hz |
|
Data acquisition & analysis: |
Empower™ Chromatography Data Software |
Lipid ASO Table
Results and Discussion
Method Considerations for HILIC of Oligonucleotides
Both the siRNA and Lipid-Conjugated ASO LC-MS Standards were analyzed using a wide-pore GTxResolve Premier BEH Amide 300 Å Column. This column is double batch-tested with both protein and oligonucleotide standards to ensure consistent, out-of-the-box performance for RNA, DNA, and viral vectors such as adeno associated viruses (AAVs). The stationary phase is packed into MaxPeak™ High Performance Surfaces (HPS) hardware to reduce nonspecific binding and eliminate the need for column passivation, enhancing reproducibility for sensitive oligonucleotide analyses. Oligonucleotides exhibit strong retention in HILIC under high organic conditions, with elution influenced by strand length, aqueous content, temperature, and ionic strength of the mobile phase.3
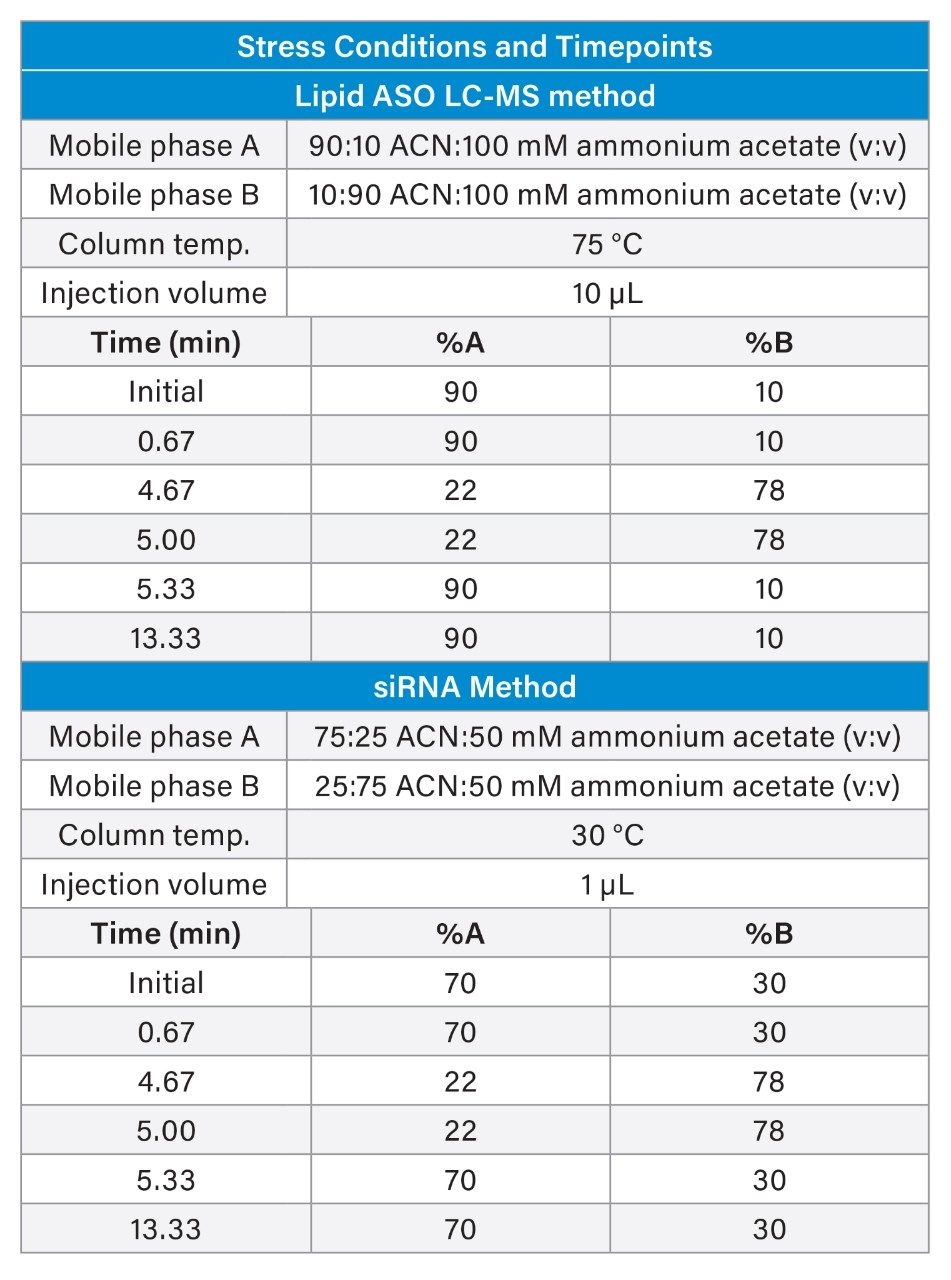
For the Lipid-Conjugated ASO LC-MS Standard, separation conditions were based on prior application notes and included high ionic strength (100 mM ammonium acetate), elevated column temperature (75 °C), and a high starting percentage of acetonitrile (82%). The elevated organic content is necessary due to the presence of a 5' palmitate modification, which increases hydrophobicity and reduces retention compared to nonlipid conjugated RNA. In contrast, the siRNA LC-MS Standard was analyzed under non-denaturing conditions with a column temperature of 30 °C—intentionally below the duplex melting temperature (Tm)—to stabilize the double stranded structure.
ASAPprime® Modeling
Chemical purity data for the oligonucleotide LC-MS standards were modeled using ASAPprime® software (version 7.0). Isoconversion times (estimated times to hit the specification limit) were used to calculate the rate k (the difference between the initial purity level and the specification limit divided by the isoconversion time) at each temperature and relative humidity condition. These k values were fit to a modified Arrhenius equation:
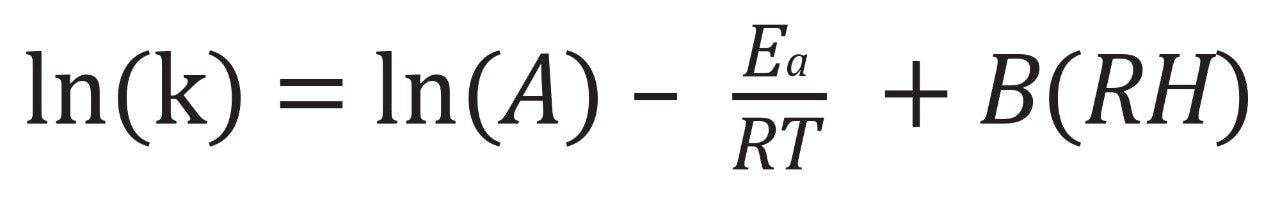
to determine the Ea (activation energy), which reflects sensitivity to temperature; B, the humidity sensitivity factor; and ln(A), the preexponential factor. These calculated Arrhenius parameters were used to determine k, and by extension the oligonucleotide shelf life, at each prospective storage condition.
Lipid-Conjugated ASO LC-MS Standard
The isoconversion plots for the Lipid-Conjugated ASO LC-MS Standard (Figure 2) depict the loss of purity over time at each temperature (25–50 °C) and relative humidity (3–29% RH) stress condition as defined in Table 1. Chromatograms of the control and a representative stressed timepoint are shown in Figure 3. The initial purity of control samples of the Lipid-Conjugated ASO LC-MS Standard was found to be 88%, and the shelf life was assessed with a specification limit corresponding to a 10% loss of purity (i.e., 78%).
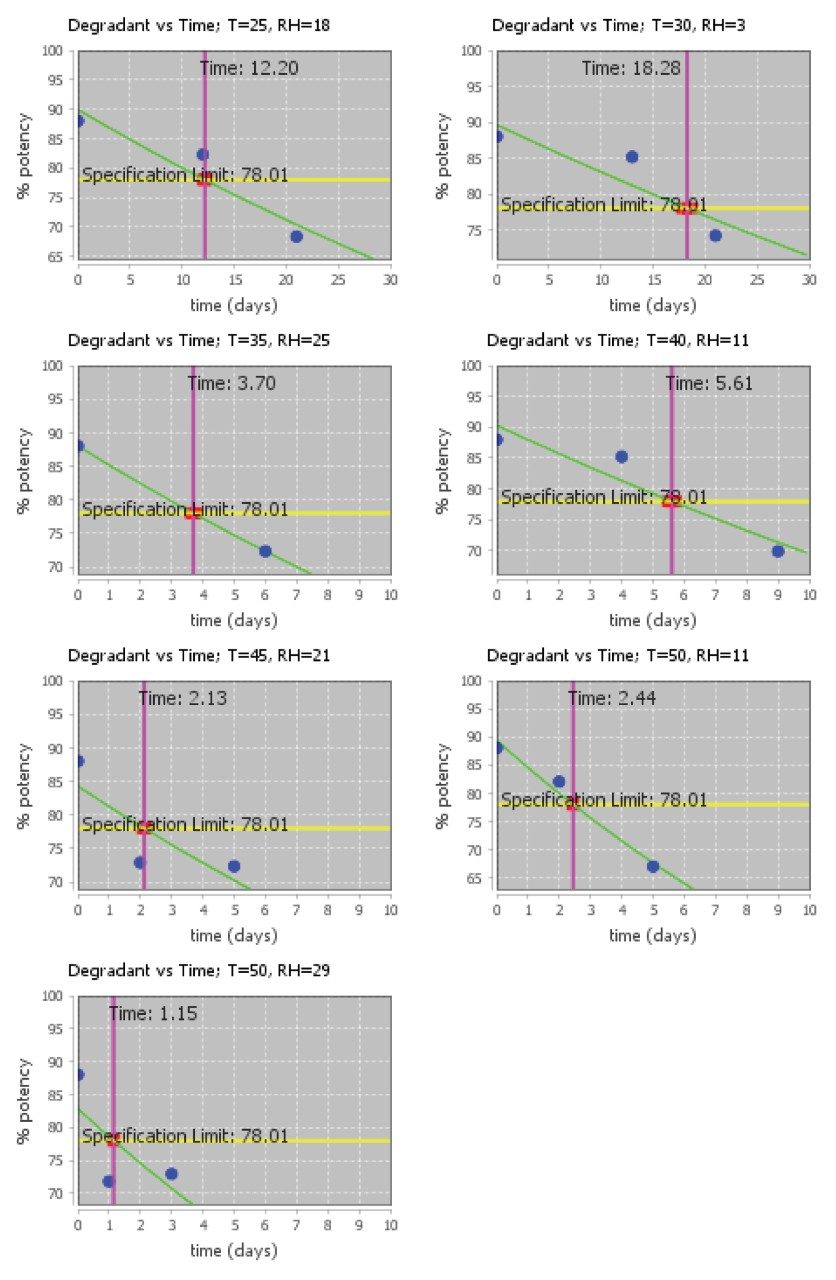
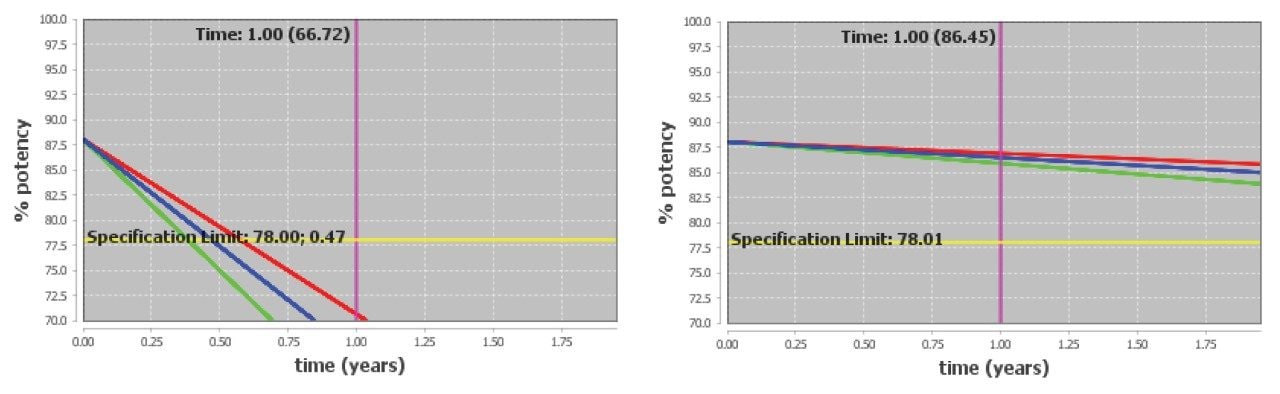
Degradation exceeded the defined specification limit under all accelerated stress conditions. Purity loss in the Lipid-Conjugated ASO LC-MS Standard exhibited both temperature sensitivity and strong moisture sensitivity: increasing relative humidity greatly increases the rate of degradation. Even assuming extremely low relative humidity inside the sealed, lyophilized vials throughout storage, the Lipid-Conjugated ASO LC-MS Standard is predicted to exceed 10% purity loss after six months of storage under refrigerated (5 °C) conditions (Figure 4). However, under frozen storage at -20 °C, the standard is expected to exhibit minimal degradation. Storage in the freezer, as recommended in the Care and Use Manual, is expected to extend the product's usable shelf life.
siRNA LC-MS Standard
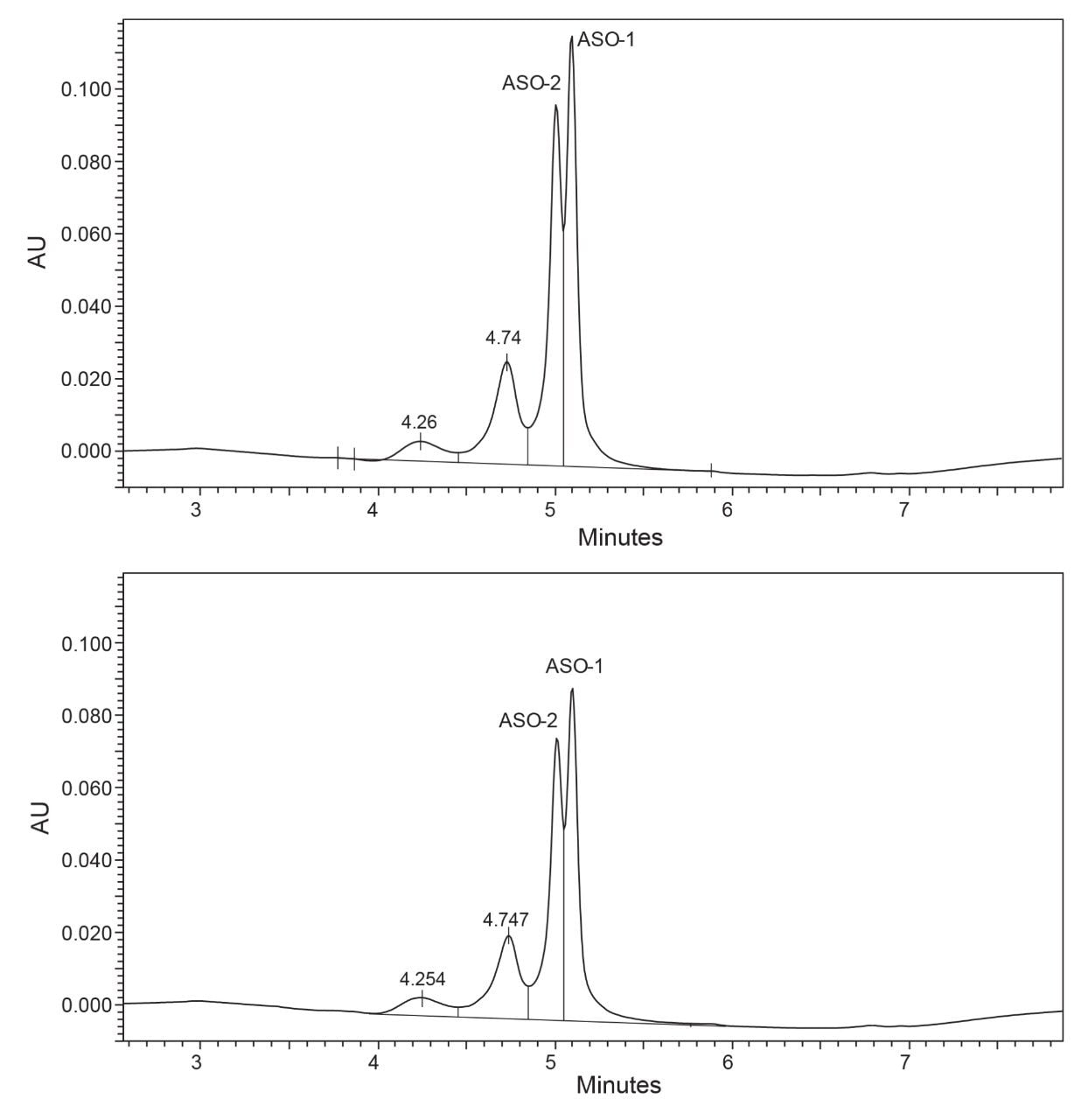
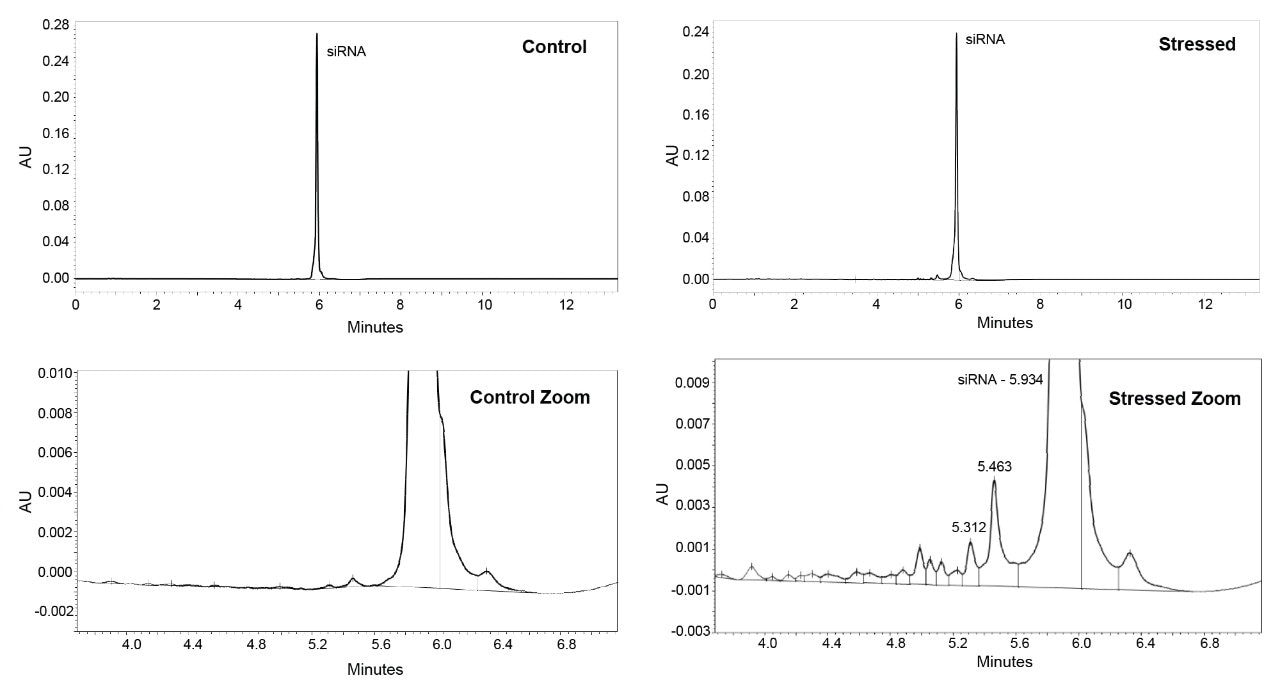
Isoconversion plots for the siRNA LC-MS Standard (Figure 5) depict the loss of purity over time based on the stress conditions shown in Table 1. The material exhibited extremely high moisture sensitivity, and small changes in the relative humidity led to a large impact on the loss of purity. Based on these results, the siRNA LC-MS Standard was stressed at milder temperature and humidity conditions than the Lipid-Conjugated ASO LC-MS Standard to generate data close to the specification limit. Chromatograms of stressed samples (Figure 6) show major impurity peaks with retention times of 5.30 and 5.45 minutes, likely corresponding to the individual RNA single strands. No degradant peaks with longer retention times appear in the chromatograms, consistent with the degradation products being from the two single strands rather than the intact duplex. The chromatographic profile supports the hypothesis that the observed extreme moisture sensitivity of the standard is due to an initial dehybridization of the siRNA duplex, followed by fast degradation of the individual single strands.
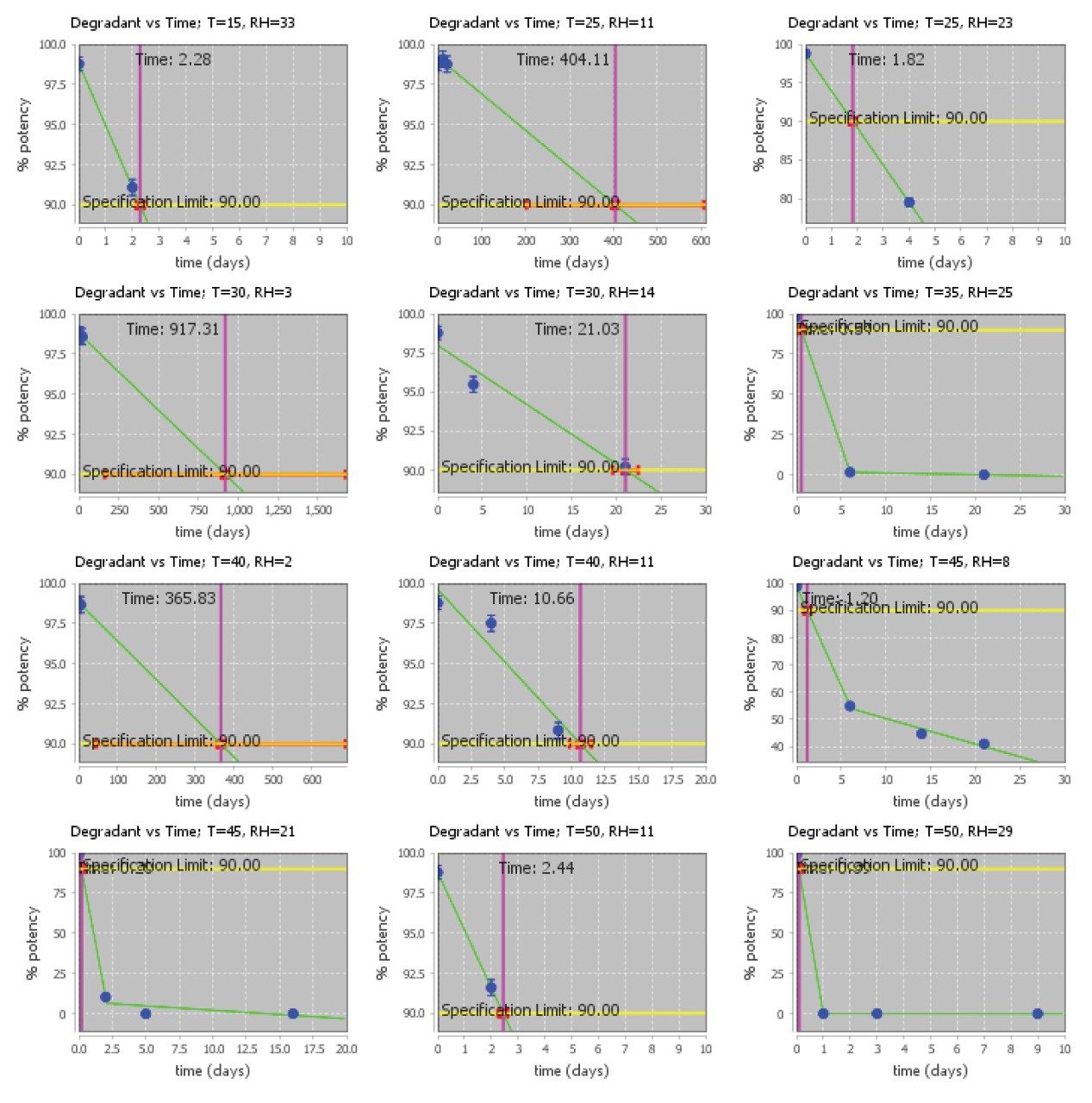
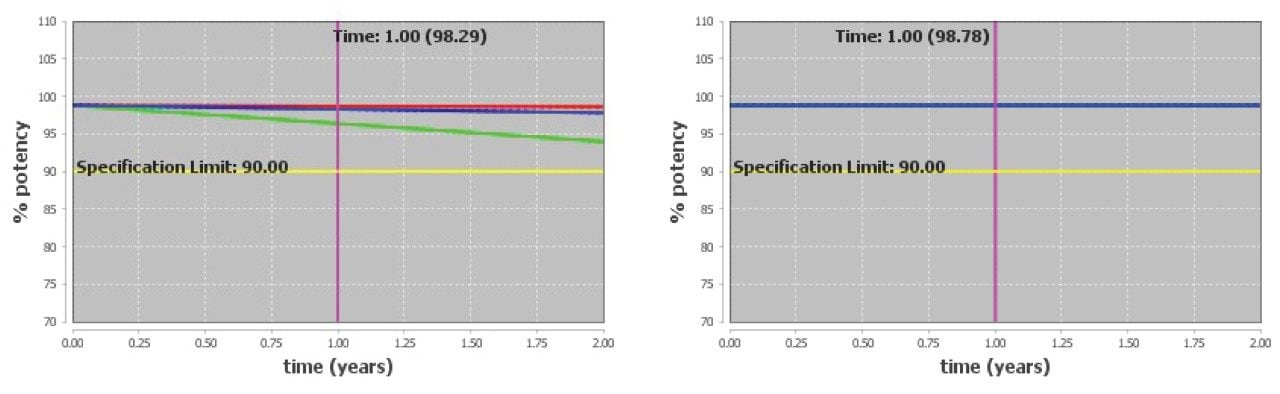
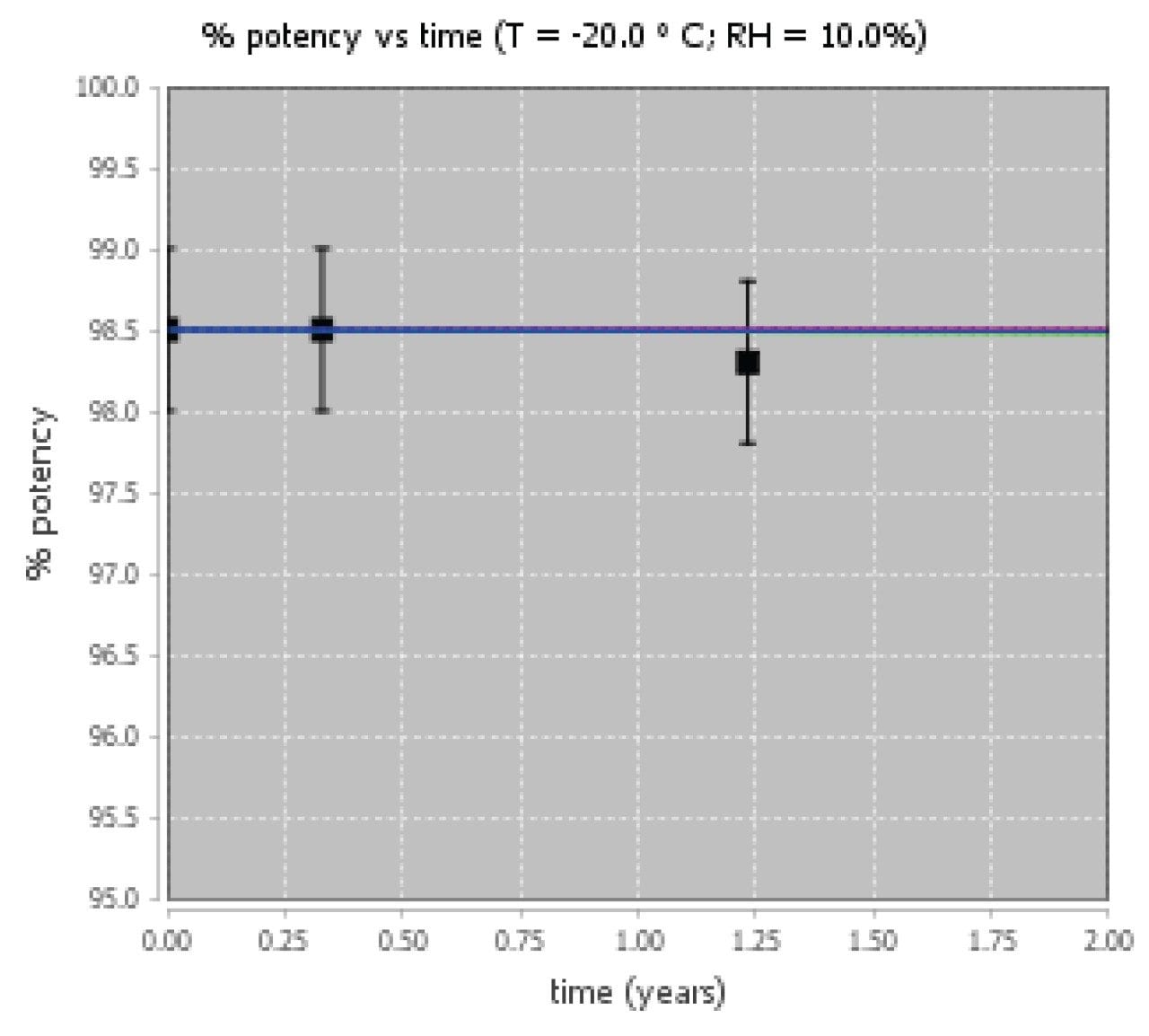
Overall, the siRNA LC-MS Standard is confidently predicted to achieve a two-year shelf life under refrigerated or frozen conditions. Example degradant plots at 5 °C/10% RH and -20 °C are shown in Figure 7. The siRNA LC-MS Standard is lyophilized and packaged at very low water activity and it is expected to be stable at these storage conditions. However, rapid degradation is expected to occur if the standard is exposed to higher humidity conditions.
Comparison to Real-Time Stability Data
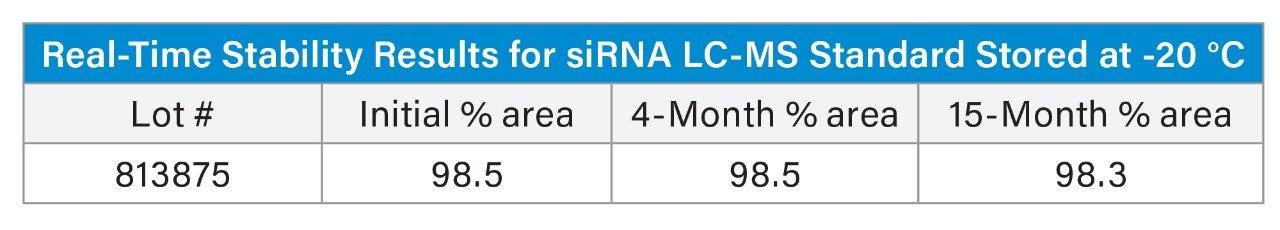
Real-time stability data for the siRNA LC-MS Standard was also collected in parallel with the ASAP study and analysis was performed by Waters Corporation. The siRNA LC-MS Standards were stored in the freezer (-20°C) and tested at 3 timepoints (initial, 4 months, and 15 months). The samples were removed and analyzed for purity based on the HILIC method outlined in the standards certificate of analysis (CofA). The ASAP model predictions for the siRNA LC-MS Standards are consistent with stability data on lot #813875, wherein no significant loss of purity is observed after fifteen months under frozen (-20 °C) conditions.
Table 2
Conclusion
ASAP studies were conducted on two distinct oligonucleotide molecules to assess their chemical stability and to demonstrate the applicability of the ASAP for RNA-based materials. Both oligonucleotide standards were successfully analyzed using the GTxResolve Premier BEH Amide Column under HILIC conditions, which provided effective separation and impurity profiling. This approach offered a streamlined and robust alternative to traditional reversed-phase ion-pairing (RP-IP) methods commonly used for oligonucleotide analysis.
Both the Lipid-Conjugated ASO LC-MS and siRNA LC-MS Standards exhibited high sensitivity to moisture under ASAP stress conditions, and strong predictive stability models were generated. The Lipid-Conjugated ASO LC-MS Standard is predicted to exceed 10% purity loss after approximately six months at 5 °C, even under very low humidity, while the siRNA LC-MS Standard is predicted to be stable at 5 °C only if very low humidity is maintained. Both standards demonstrated strong stability under frozen storage, consistent with lyophilized products and Care and Use Manual guidance. For the siRNA LC-MS Standard, this prediction is further supported by real-time data showing no significant purity loss over 15 months at -20 °C.
Based on the humidity sensitivity identified through ASAP modeling, a molecular sieve has been incorporated into the sealed foil packaging of the siRNA LC-MS Standard and Lipid-Conjugated ASO LS-MS Standard. A molecular sieve is a highly porous desiccant that selectively absorbs moisture from the surrounding environment, helping to maintain the low moisture activity. This addition provides protection with the intent to further minimize the risk of humidity-driven degradation during storage and handling.
References
- Waterman K.C., Swanson J.T., Lippold B.L. Scientific and Statistical Analysis of Accelerated Aging for Pharmaceuticals. Part 1: Accuracy of Fitting Methods. J Pharm Sci, 2014, 103(10), 3000–3006.
- Li Y. and Breaker R.R. Kinetics of RNA Degradation by Specific Base Catalysis of Transesterification Involving the 2’-Hydroxyl Group. J Am Chem Soc, 1999, 121, 5364–5372.
- Finny A.S., Johnston T, Sauner B., Addepalli B, Lauber M.A. Reproducible Hydrophilic Interaction Chromatography for Denaturing and Non-Denaturing Analyses of Oligonucleotides Using GTxResolve Premier BEH Amide Columns. Waters Application Note, 720008456. August, 2024.
720008945, August 2025