Metrological Traceability of the Waters™ MassTrak™ Steroid Serum Set 2 and 3 Calibrators and Quality Controls
Ce document est une note d’application et ne contient pas de section détaillée concernant l’expérimentation.
Pour usage diagnostique in vitro. La disponibilité varie selon les pays.
Abstract
Clinical laboratories utilizing LC-MS/MS methods are often obligated to validate their test methods to comply with the regulatory body in their region. In some geographies, laboratories are required to use metrologically traceable calibration materials to aid in compliance with ISO 15189–2022 (Medical Laboratories- Requirements for quality and competence), to ensure their calibration material is metrologically traceable. Therefore, Waters incorporates metrological traceability of all its analytes into the design, development and manufacture of their MassTrak Steroid Serum Calibrator and Quality Control Sets and providing confidence in the accuracy and harmonization of results when using validated LC-MS methods.
In this application note, we provide a summary of accuracy and lot-to-lot testing carried out on MassTrak Steroid Serum Calibrators Set 2 and 3, alongside a step-by-step guide on how Waters ensures the metrological traceability of these products.
Benefits
- Metrologically traceable calibrators and QCs, ensures compliance with ISO regulations (ISO 15189)
- Confidence in the accuracy and precision of primary and secondary standards when compared with reference laboratories
- Lyophilized products, reduce sample preparation time
Introduction
The use of liquid chromatography - mass spectrometry has become a routine analysis technique employed by a variety of laboratories in recent years, with a shift away from ELISA based methods for a more robust, sensitive, selective, and reliable workflow. With this shift comes more manual preparation as many clinical laboratories methods are based on laboratory developed tests (LDTs), in-house calibrators and QCs may be prepared and characterized by laboratory staff, which can lead to inaccuracies and a reduction in productivity within the laboratory. This is compounded when regional ISO guidelines such as ISO 15189–2022 must be adhered to. Hence the need for metrologically traceable LC-MS/MS calibrators and QCs, manufactured to a high standard to ensure customer confidence and reduce variability across results.
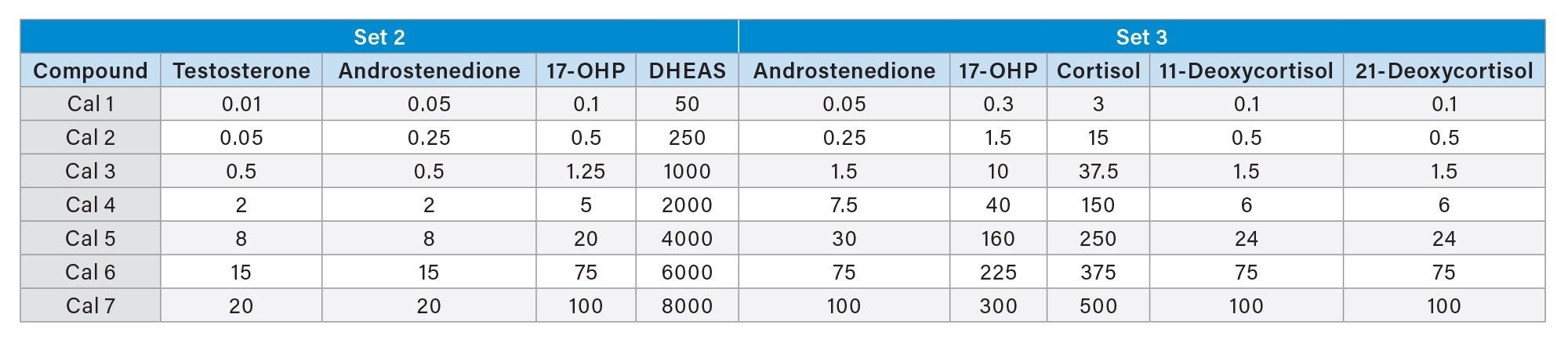
The MassTrak Steroid Serum Sets 2 and 3 (IVDR) contain a range of steroid hormones in lyophilized serum which are metrologically traceable to the highest level of metrologically traceable standards available. Calibrator Set 2 contains androstenedione, testosterone, 17-hydroxyprogesterone (17-OHP), and dehydroepiandrostenedione-sulfate (DHEAS) and is intended for the quantitative determination of androgens and progestogens in human serum to aid with monitoring of physiological markers. While the MassTrak Steroid Serum Calibrator Set 3 contains androstenedione, 17-OHP, cortisol, 11-deoxycortisol, and 21-deoxycortisol and is intended to be used for the quantitative determination of androgens and glucocorticoids in human serum to aid with monitoring of physiological markers. All the Waters MassTrak Steroid Serum sets are value assigned using metrologically traceable primary and secondary in-house standards, which are prepared using certified reference materials in ultra-low hormone and steroid charcoal stripped serum. The concentrations of these in house standards are independently confirmed using independent QC material and EQA schemes, where available.
Results and Discussion
Metrological Traceability
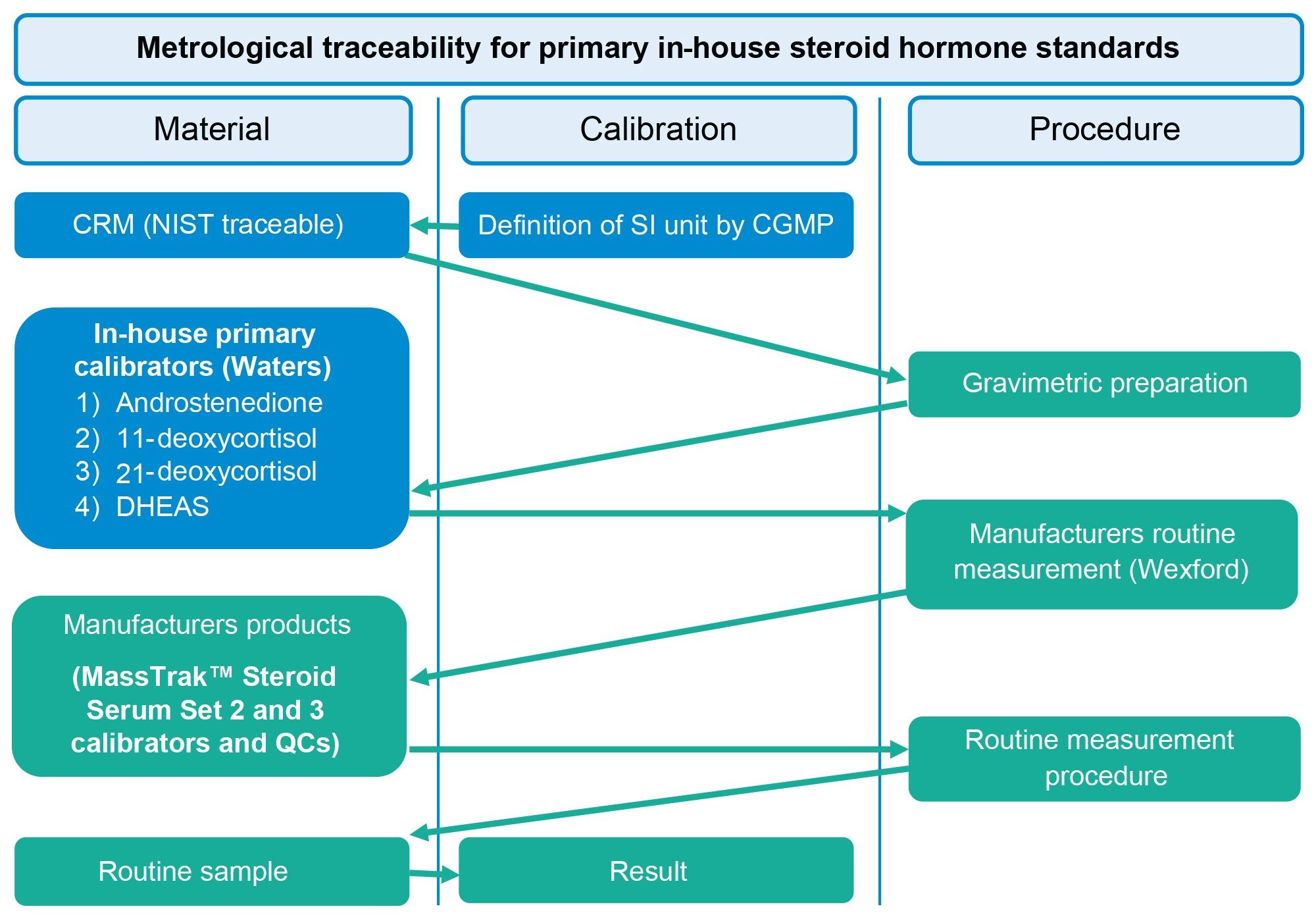
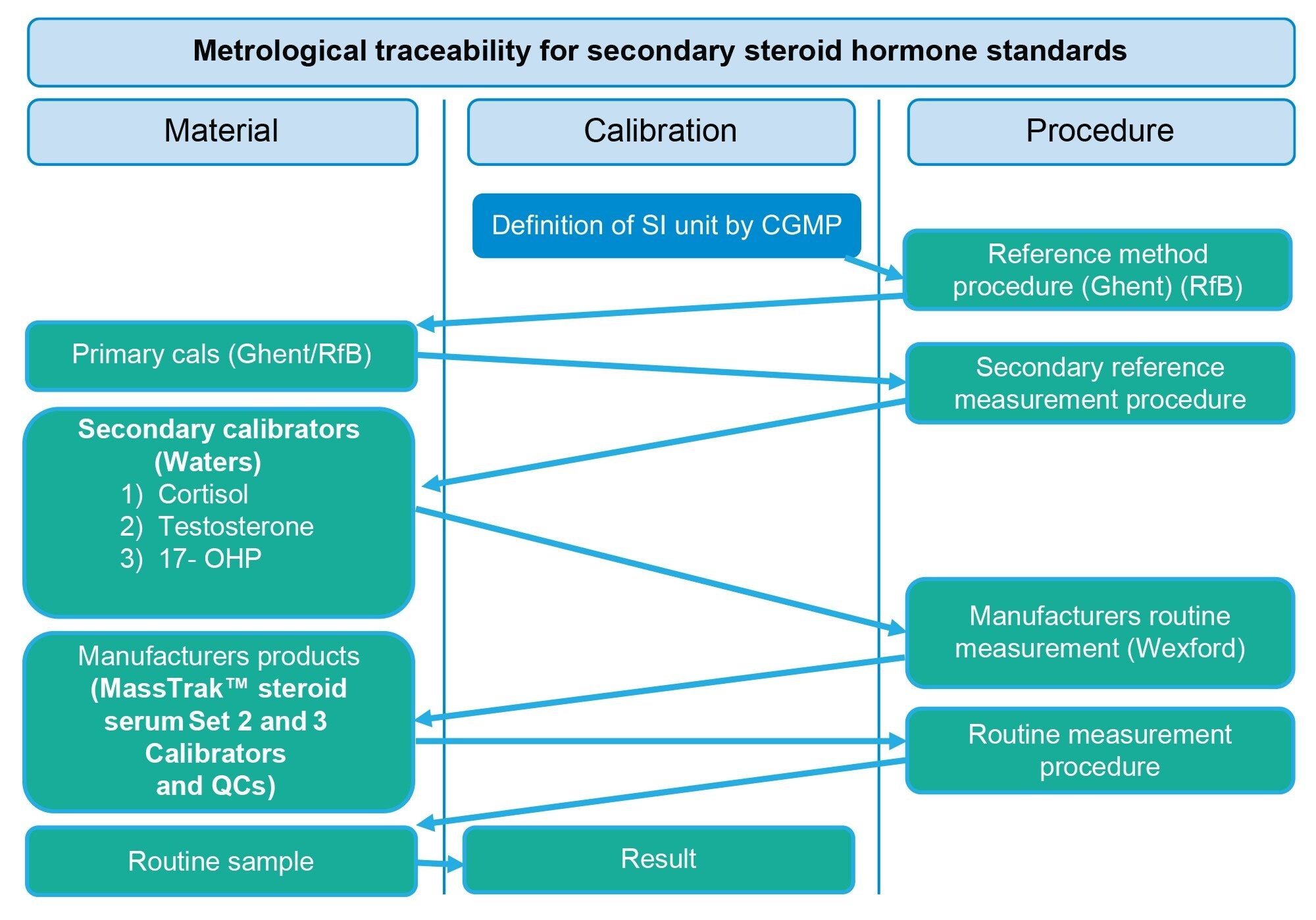
Two metrological traceability processes are employed for the assignment of the steroid calibrators and QCs, in the form of primary in-house standards and secondary in-house standards. Primary in-house standards are gravimetrically prepared, are NIST traceable and contain the following analytes, androstenedione, DHEAS, 11-deoxycortisol, 21-deoxycortisol (Figure 2). Secondary in-house standards, containing testosterone, cortisol, and 17-OHP (Figure 3), which are traceable to the University of Ghent and Referenzinstitut für Bioanalytik (RfB), are value assigned using reference measurement procedures, with values based on a secondary reference measurement procedure assignment. These primary and secondary standards are then used to generate and assign concentrations of the MassTrak Steroid Serum Calibrator and QC Sets, which are subsequently confirmed using independent quality controls in the form of EQA material and in-house panels.
Comparison Between Waters and Reference Laboratories
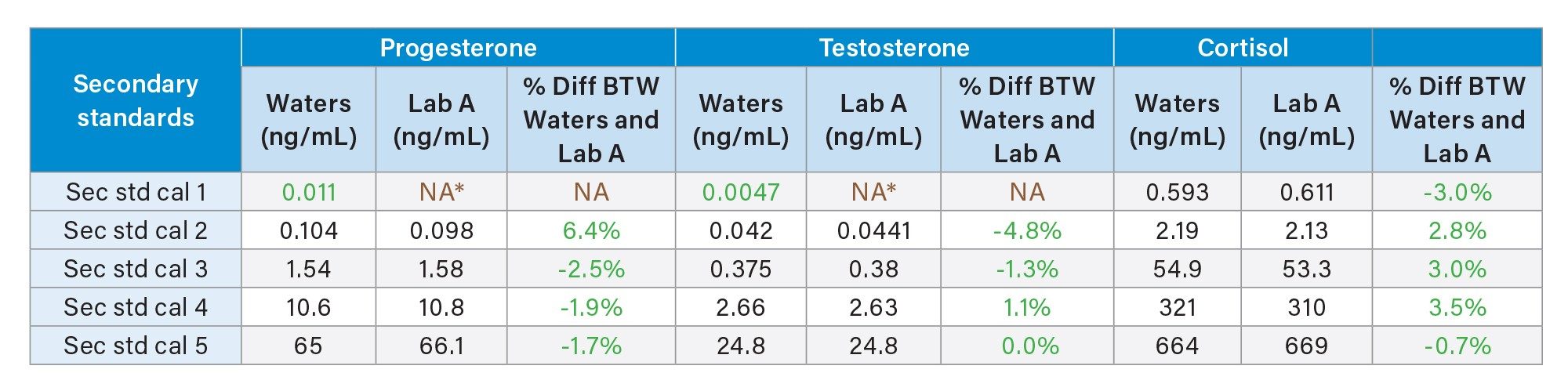
The secondary standards used to generate and assign concentrations to the MassTrak Steroid Serum Calibrators and Quality Controls, are prepared and internally value assigned using Waters value assignment procedure, to ensure the values are within procedural specification before being sent to metrologically traceable reference laboratories for value assignment using reference measurement procedures (outlined in Table 1).
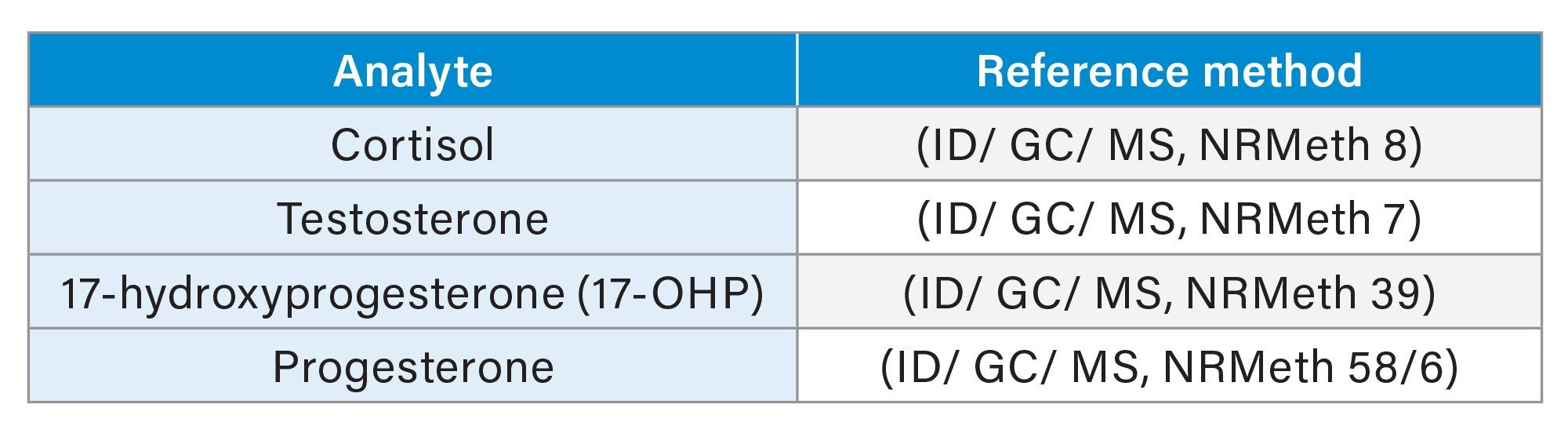
It should be noted that progesterone is not contained in Set 2 and 3 cals and QCs, this information is for demonstration purposes only. Refer to application note “Metrological Traceability of the Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets” –720007404, which contains metrological traceability information pertaining to MassTrak Steroid Serum Set 1 Calibrators and QCs, which contains progesterone, cortisol, testosterone, 17-OHP, DHEAS, 21-deoxycortisol, corticosterone, 11-deoxycortisol, androstenedione, 11-deoxycorticosterone, dehydroepiandrosterone (DHEA), and dihydrotestosterone (DHT).
Table 2 displays the results of Waters internal value assignment of secondary standards to the reference measurement provided by the reference laboratory (Lab A). It has been highlighted in table 2, however the concentration of secondary standard calibrator 1 is lower than the reference laboratories Lower Limit of Quantification* (LLoQ*) for progesterone and testosterone. When Waters is compared to Lab A, for calibrators 2–5 there is marginal deviation between the value assignments, giving Waters confidence in using our value assignments for calibrator 1. The max % difference for cortisol is 3.5%, progesterone 6.4% and 4.8% for testosterone. These results provide further assurance and confidence in the MassTrak Steroid Serum Calibrators and QCs, accurately ensuring comparable patient results from different lots of products.
Accuracy and Lot to Lot Comparison
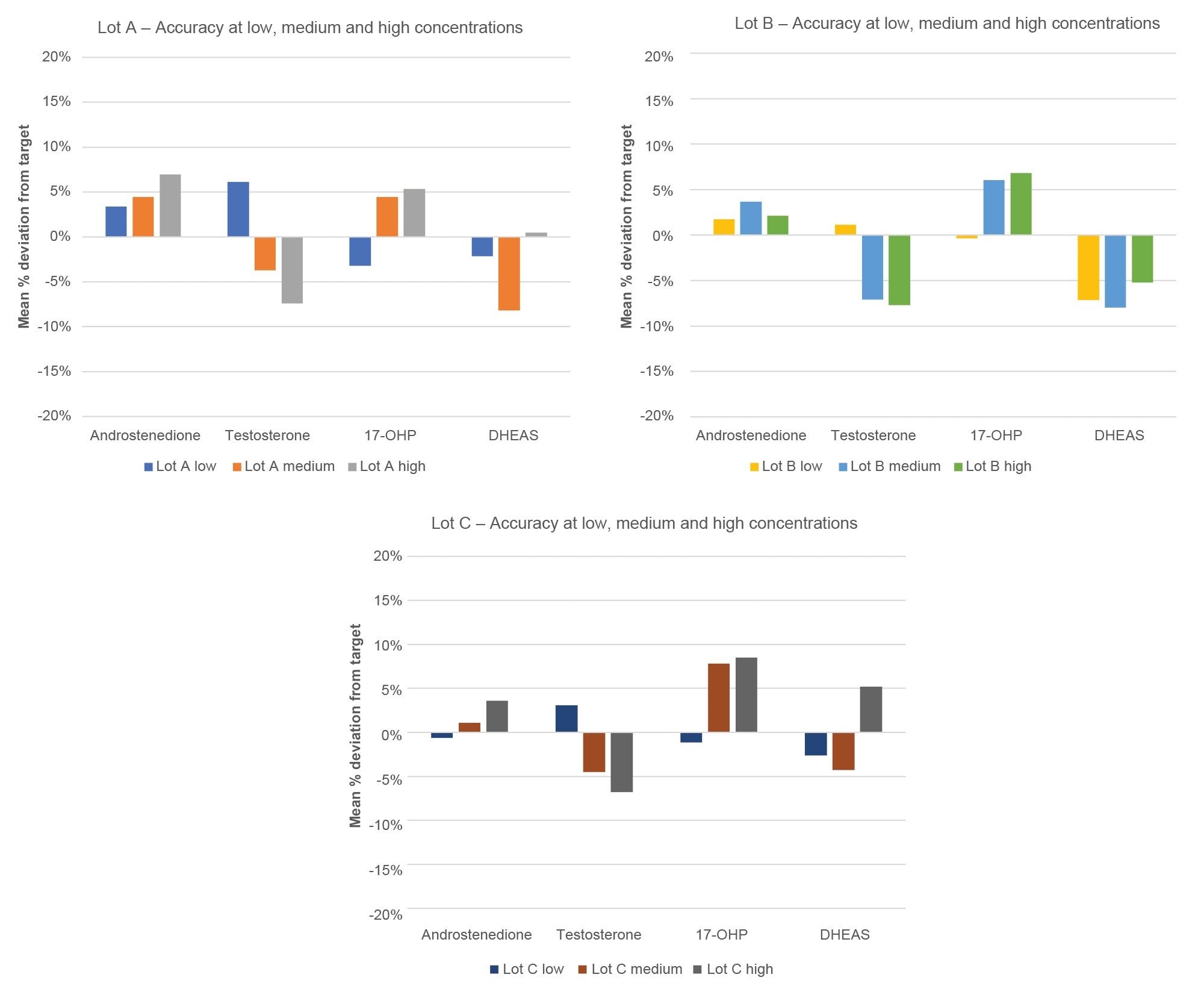
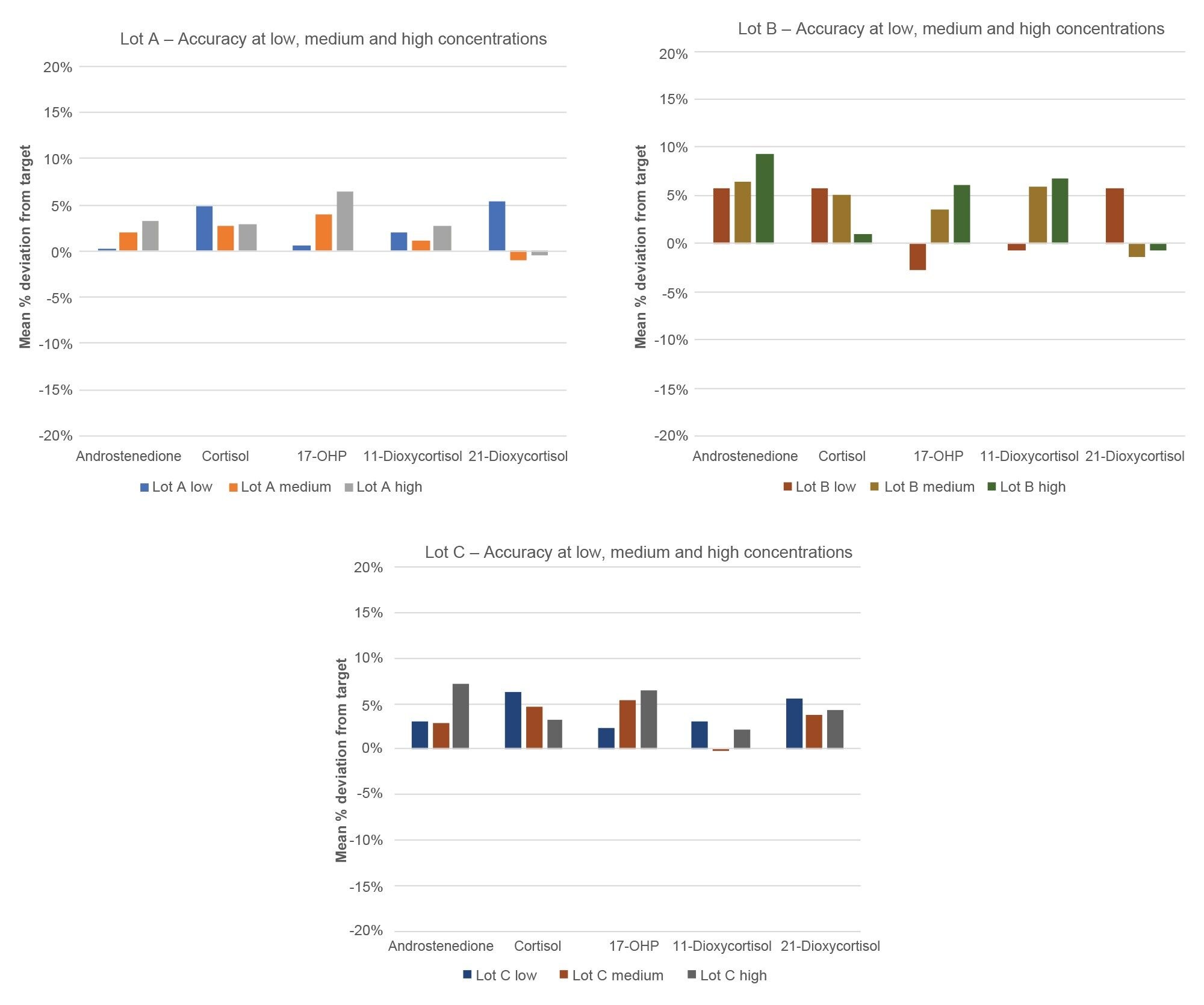
Accuracy of the calibrator sets was assessed for testosterone, androstenedione, 17-OHP, and cortisol through the analysis of EQA material. Data was obtained across three different manufacturing lots by accessing the accuracy in measuring at the low, medium, and high end of the calibration line for each MassTrak Steroid Serum Calibrator Set. These results were then compared to the mass spectrometry assigned target value for each EQA sample and the mean % difference from target was tabulated. Due to the unavailability of EQA material for 11-deoxycortisol and 21-deoxycortisol, in-house panels, which are NIST traceable, were used to assess the accuracy of these compounds at the low, medium, and high range. Results are shown in Figures 4 and 5, this data demonstrates the accuracy of the calibrator sets within ± 10% but also the lot-to-lot reproducibility of the manufacturing process which is imperative for clinical laboratories to have a consistent and reliable testing materials.
Measurement Uncertainty
The measurement uncertainty for the MassTrak Steroid Serum Set 2 and 3 calibrators were calculated using the following measurement data; accuracy, precision, the expanded uncertainty of material, and the laboratory equipment used.
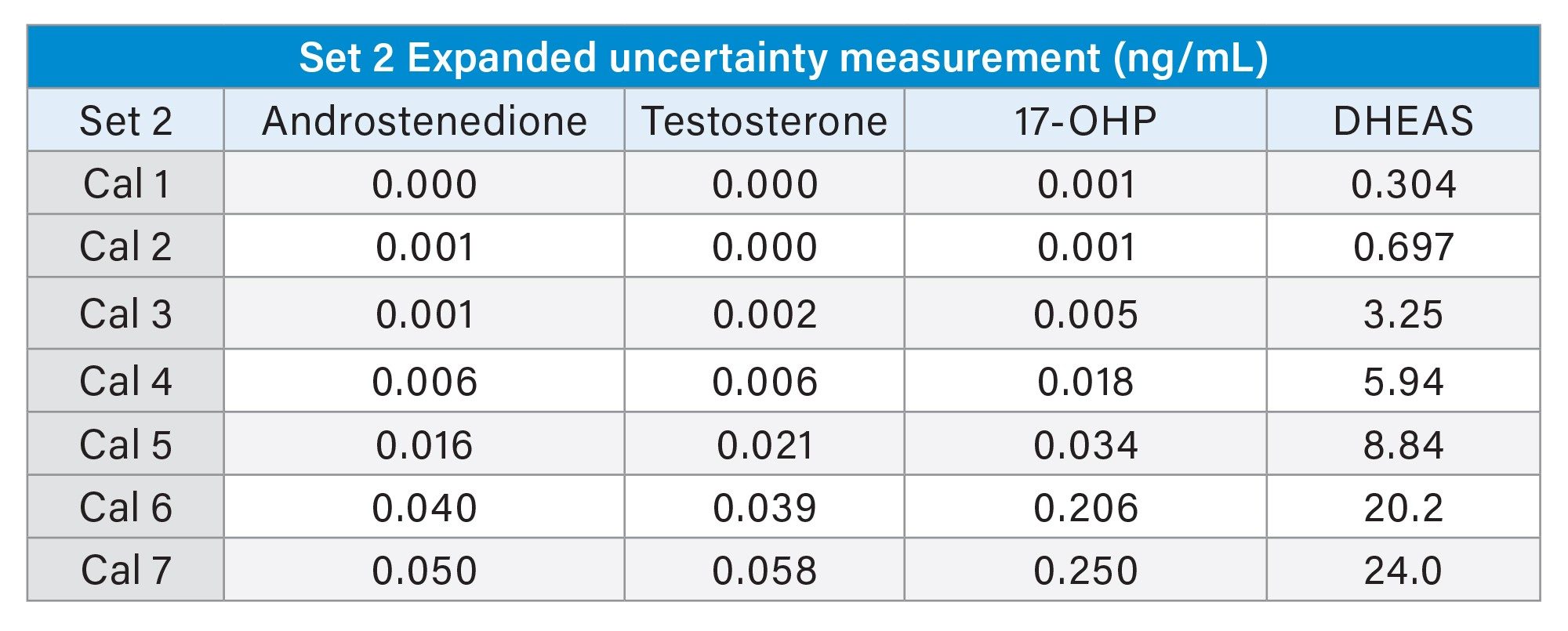
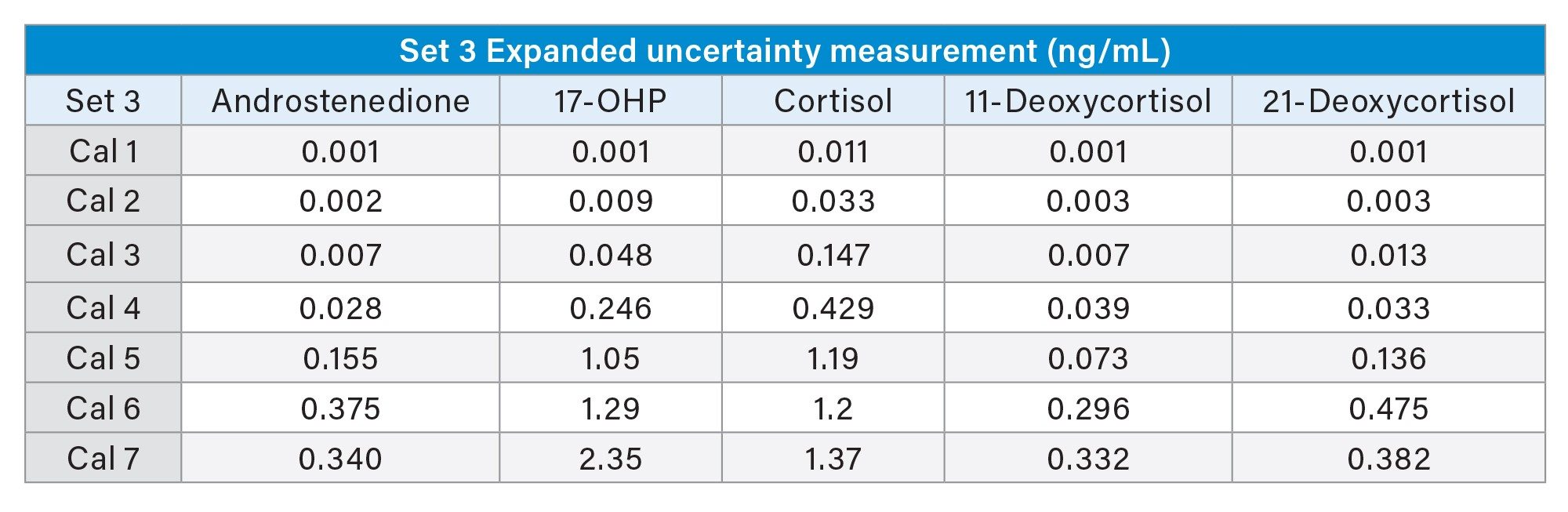
Conclusion
The metrological traceability of the MassTrak Steroid Serum Set 2 and 3 cals and QCs has been confirmed through the use of primary and secondary in-house standards, aiding laboratories compliance to ISO 15189. The precision and accuracy of our products were confirmed through the measurement of independent QC material in the form of EQA material supplied by UK NEQAS.
References
- Foley D, Rossiter P, Breen N, Calton, LJ. Metrological Traceability of the Waters MassTrak Endocrine Steroid Calibrator and Quality Control Sets. Waters Application Note 720007404. 2022 Apr.
- Pitt J. Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry. The Clinical Biochemist Reviews. 2009 Feb; 30(1): 19–34.
Featured Products
720008223, February 2024