Confidence in Your Calibrators: MassTrak™ Immunosuppressant Calibrator and Quality Control Sets for the LC-MS/MS Analysis of Cyclosporine, Everolimus, Sirolimus, and Tacrolimus
For in vitro diagnostic use. Not available in all countries.
Abstract
The immunosuppressive drugs cyclosporine, everolimus, sirolimus and tacrolimus have historically been measured using immunoassay. However variable accuracy at low concentrations coupled with specificity issues due to cross-reactivity of antibodies with other components, such as metabolites, can cast doubt on results. This phenomenon is well documented in the literature. As such, many clinical laboratories increasingly analyse these drugs using liquid chromatography with tandem mass spectrometry (LC-MS/MS), for which they require reliable, reproducible calibrators, and quality control sets (QCs) for confidence in their results.
Waters™ MassTrak Immunosuppressant Calibrator and QC Sets (IVD) provide confidence in the accuracy and aid harmonization of results when using validated LC-MS/MS methods.
The MassTrak Immunosuppressant Calibrators and Quality Control Sets performance was demonstrated using the ACQUITY™ UPLC™ I-Class FL and Xevo™ TQ-S micro Triple Quadrupole Mass Spectrometer and an in-house developed LC-MS/MS methodology.
Benefits
- Guiding principles described in ISO17511 adhered to for value assignment
- Confidence in the accuracy of immunosuppressants and provides a path to laboratory method harmonization
- Lyophilized calibrators and QCs that reduce sample preparation time
Introduction
Routine analysis of immunosuppressant drugs in whole blood by immunoassay is highly automatable and affords analytical sensitivity, however issues relating to selectivity and multiplexing remain. As such, reliability of results, particularly at low concentrations can be a concern. Latterly, LC-MS/MS has become increasingly more-widely adopted for immunosuppressant analysis in order to overcome these known limitations.
LC-MS/MS methods in clinical laboratories are often based on laboratory developed tests (LDTs), validated to local regulatory guidelines. These guidelines are constantly evolving and there is increasing demand for all aspects of clinical methods to comply with these changing regulations. This includes the calibrator and QC materials used to generate and independently check the accuracy of the calibration within the method.
The Waters MassTrak Immunosuppressant Calibration and Quality Control Sets (IVD) (Figure 1) contains cyclosporine, everolimus, sirolimus, and tacrolimus in lyophilized whole blood that have been sourced to obtain the highest level of metrological traceability available. In order to demonstrate the quality of materials found in this product, we have shown the proof-of-concept performance of the materials using protein precipitation and separation and detection of the samples using the ACQUITY UPLC I-Class FL with Xevo TQ-S micro Triple Quadrupole Mass Spectrometer.
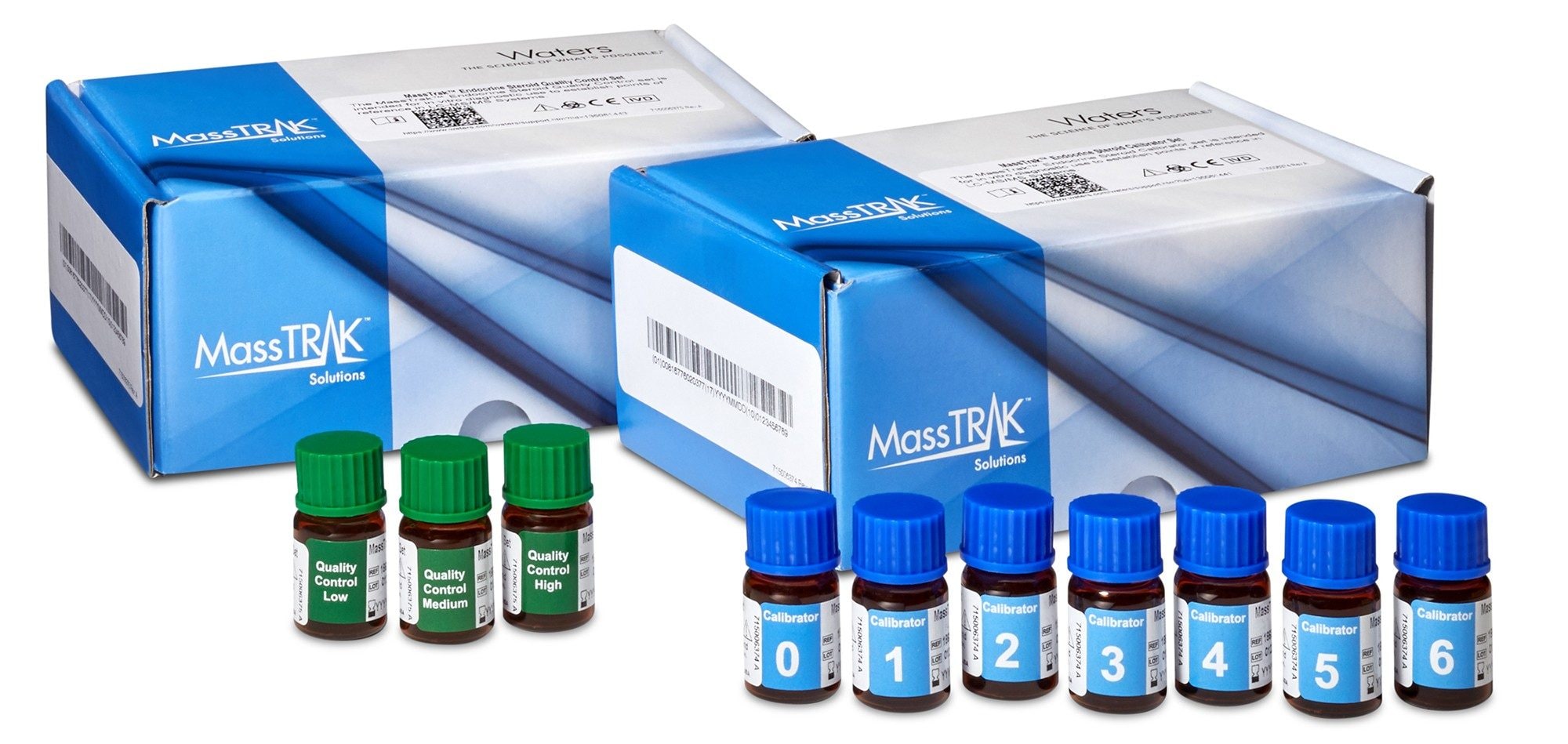
Experimental
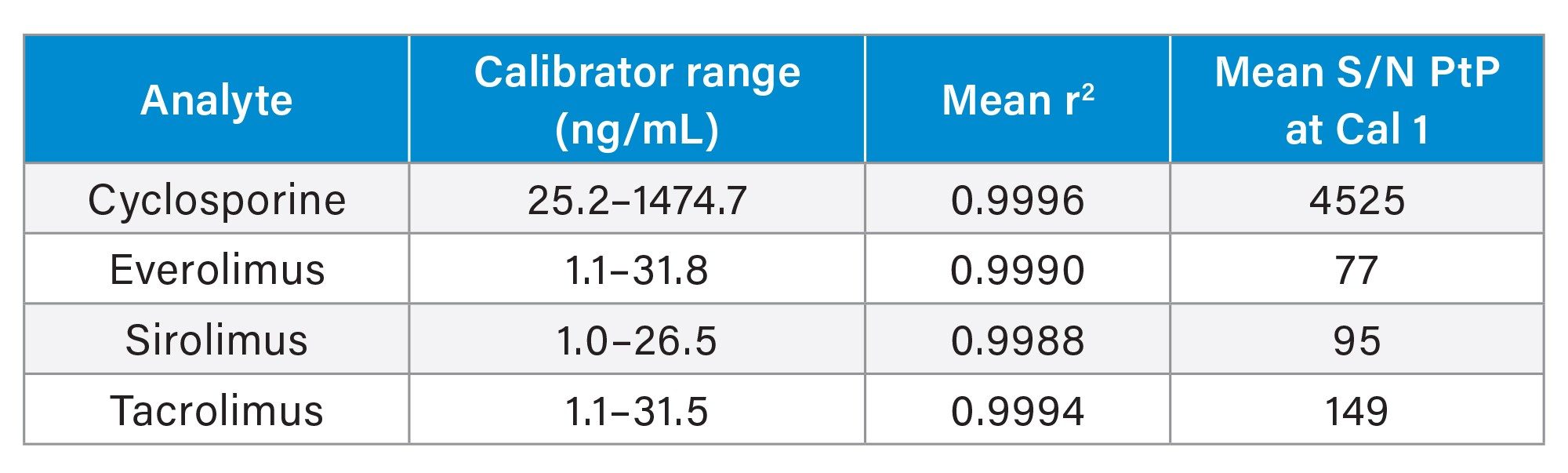
The Waters MassTrak Immunosuppressant Calibration and Quality Control Sets contain the following immunosuppressant drugs in lyophilized whole blood: cyclosporine, everolimus, sirolimus, and tacrolimus. Assigned concentrations for the calibration range and QCs are found in Table 1.
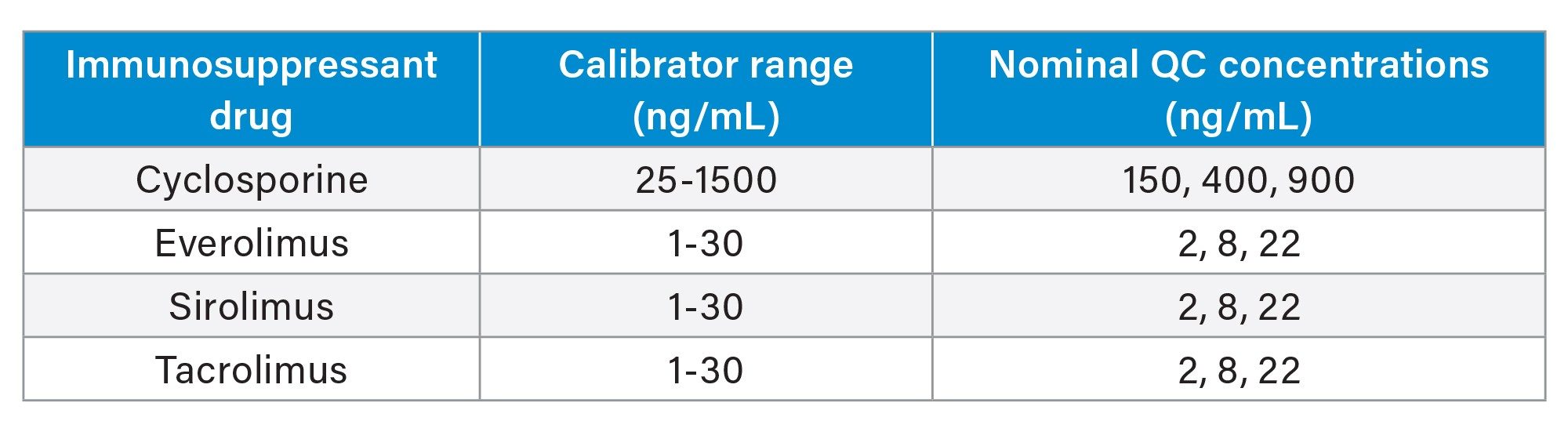
Sample Description:
Sample preparation was performed by using protein precipitation
Protein Precipitation:
To 50 µL of whole blood sample, 200 µL of 0.1 M aqueous zinc sulfate was added and mixed for five seconds. 500 µL of internal standard (ISTD) was added, followed by mixing for twenty seconds. Samples were then centrifuged for two minutes at 4696 g.
LC Conditions:
|
System: |
ACQUITY UPLC I-Class with FL |
|
Needle: |
20 µL |
|
Loop: |
50 µL |
|
Column: |
ACQUITY UPLC HSS C18 SB Column; 1.8 µm, 2.1 x 30 mm (p/n: 186004117) |
|
Column temp.: |
55 °C |
|
Sample temp.: |
8 °C |
|
Injection volume: |
20 µL |
|
Injection mode: |
PLNO, with Load Ahead enabled |
|
Mobile phase A: |
Water + 2 mM ammonium acetate + 0.1% formic acid |
|
Mobile phase B: |
Methanol + 2 mM ammonium acetate + 0.1% formic acid |
|
Weak wash: |
Water:methanol 95:5 (v:v), 600 µL |
|
Strong wash: |
Water:methanol:acetonitrile:IPA 1:1:1:1 (v:v:v:v), 200 µL |
|
Seal wash: |
Water:methanol 80:20 (v:v) |
Gradient Table
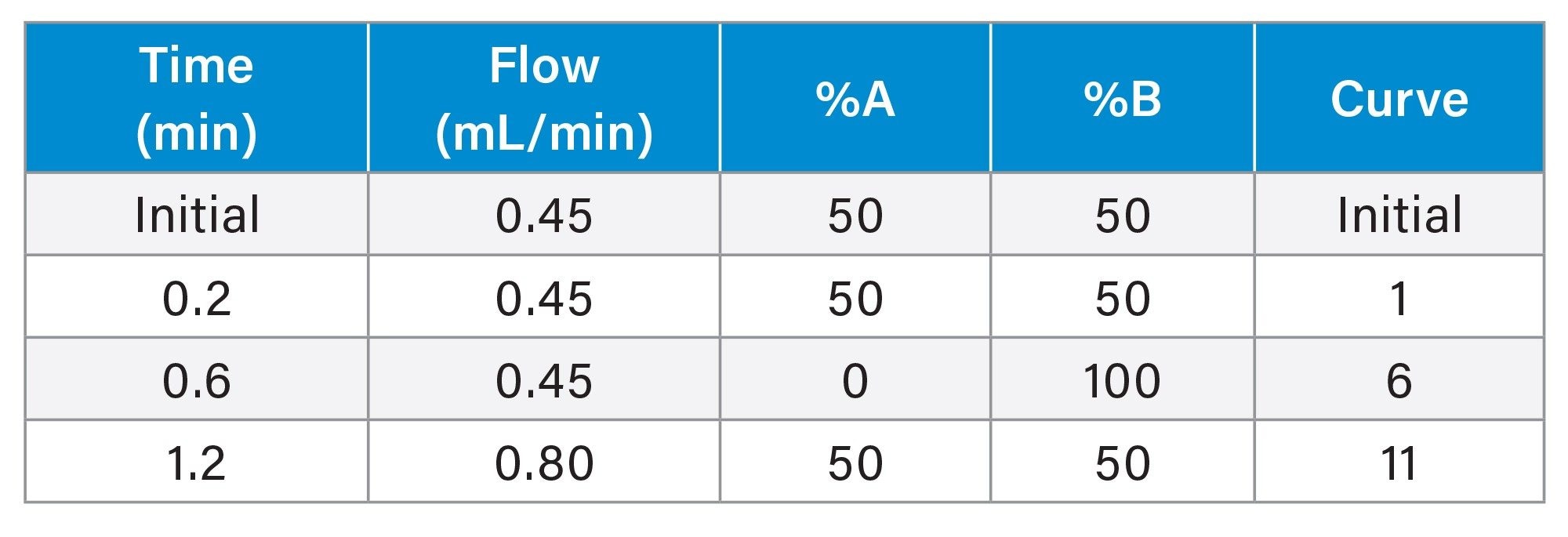
MS Conditions
|
MS system: |
Xevo TQ-S micro Triple Quadrupole Mass Spectrometer |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.8 kV |
MRM Parameters
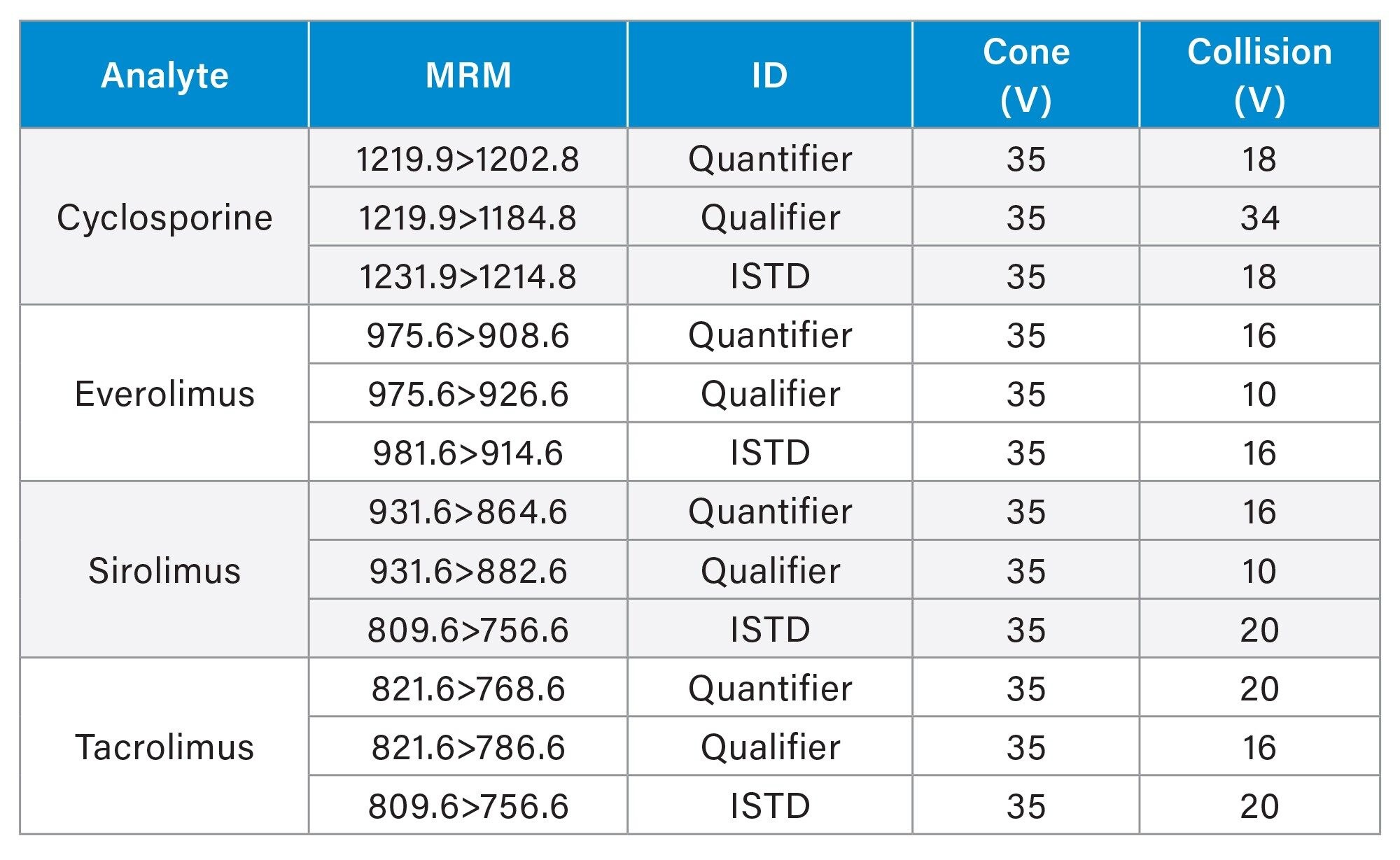
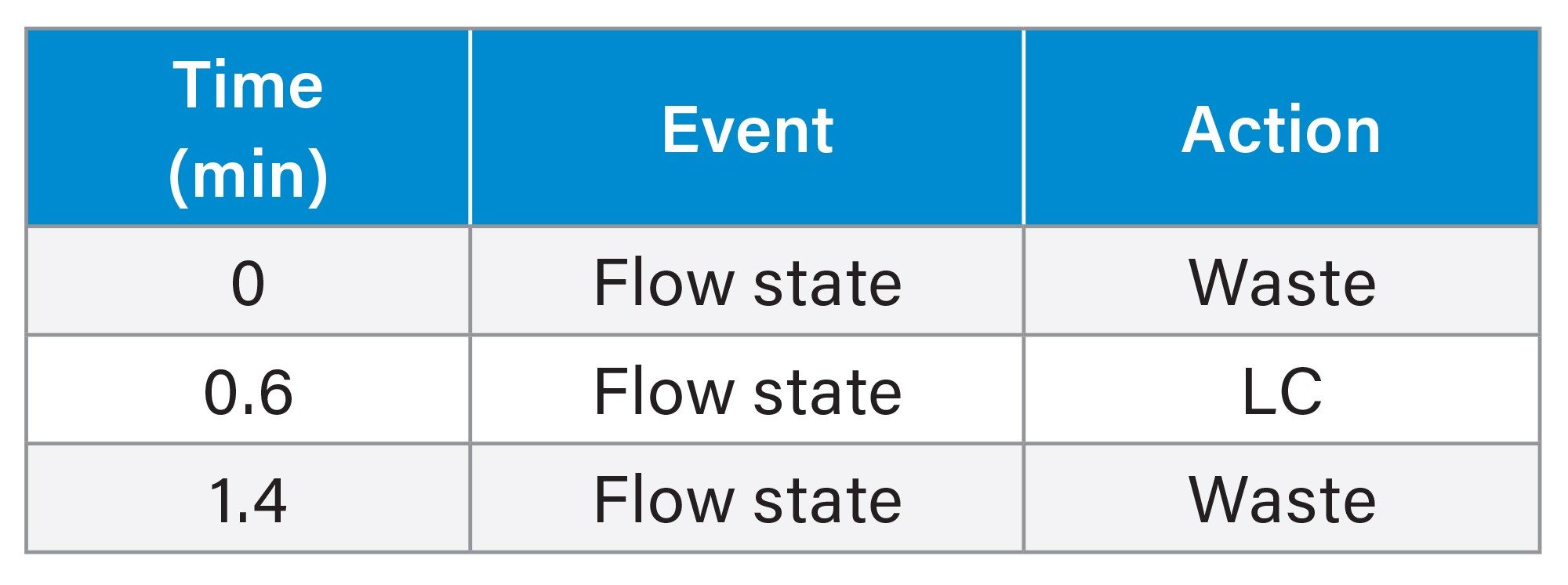
Method Events
Results and Discussion
The four immunosuppressive drugs were chromatographed using the ACQUITY UPLC 2.1 mm x 30 mm HSS C18 SB Column.
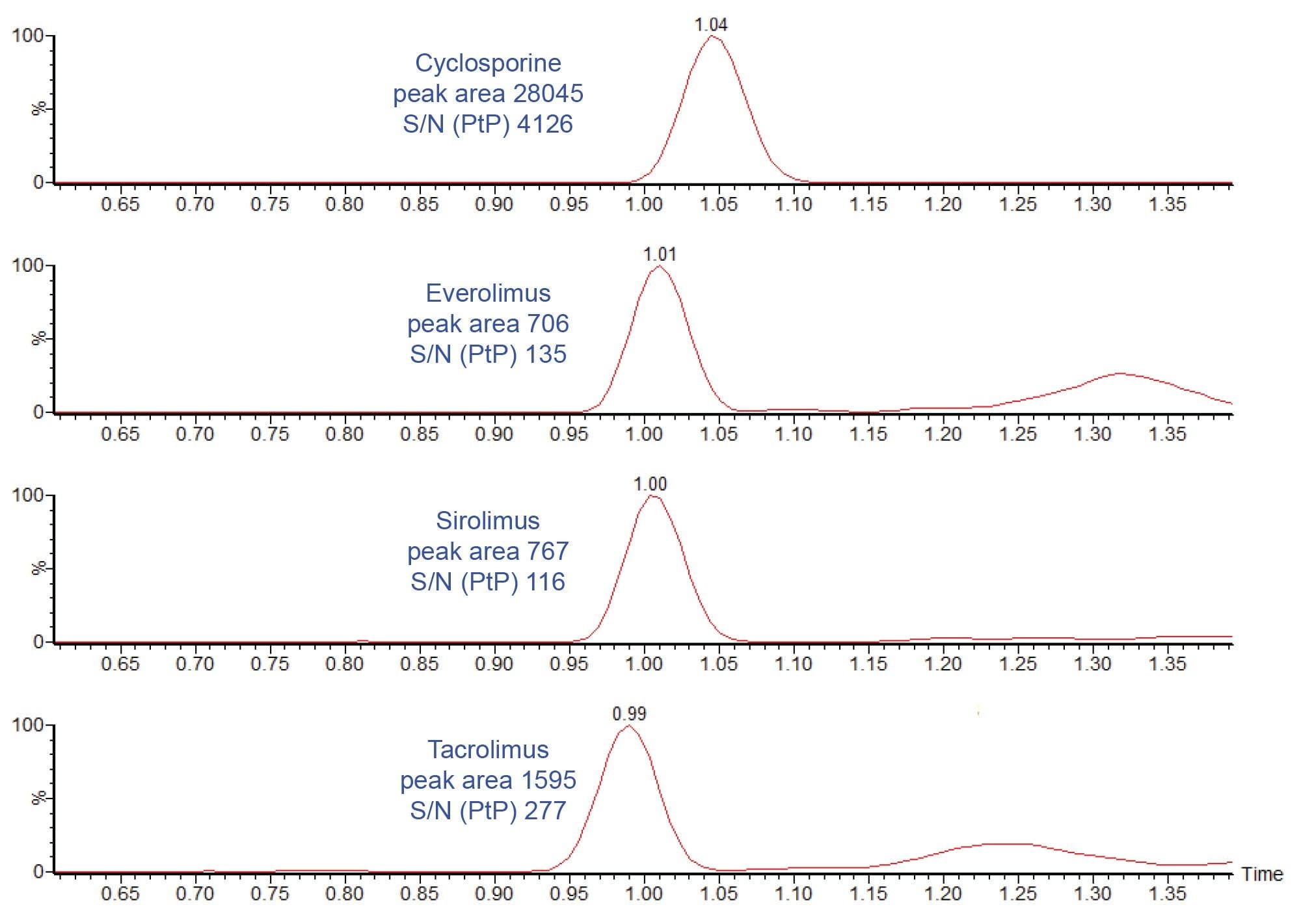
Figure 2 shows an example chromatogram of calibrator 1 (25 ng/mL cyclosporine and 1 ng/mL everolimus, sirolimus, and tacrolimus).
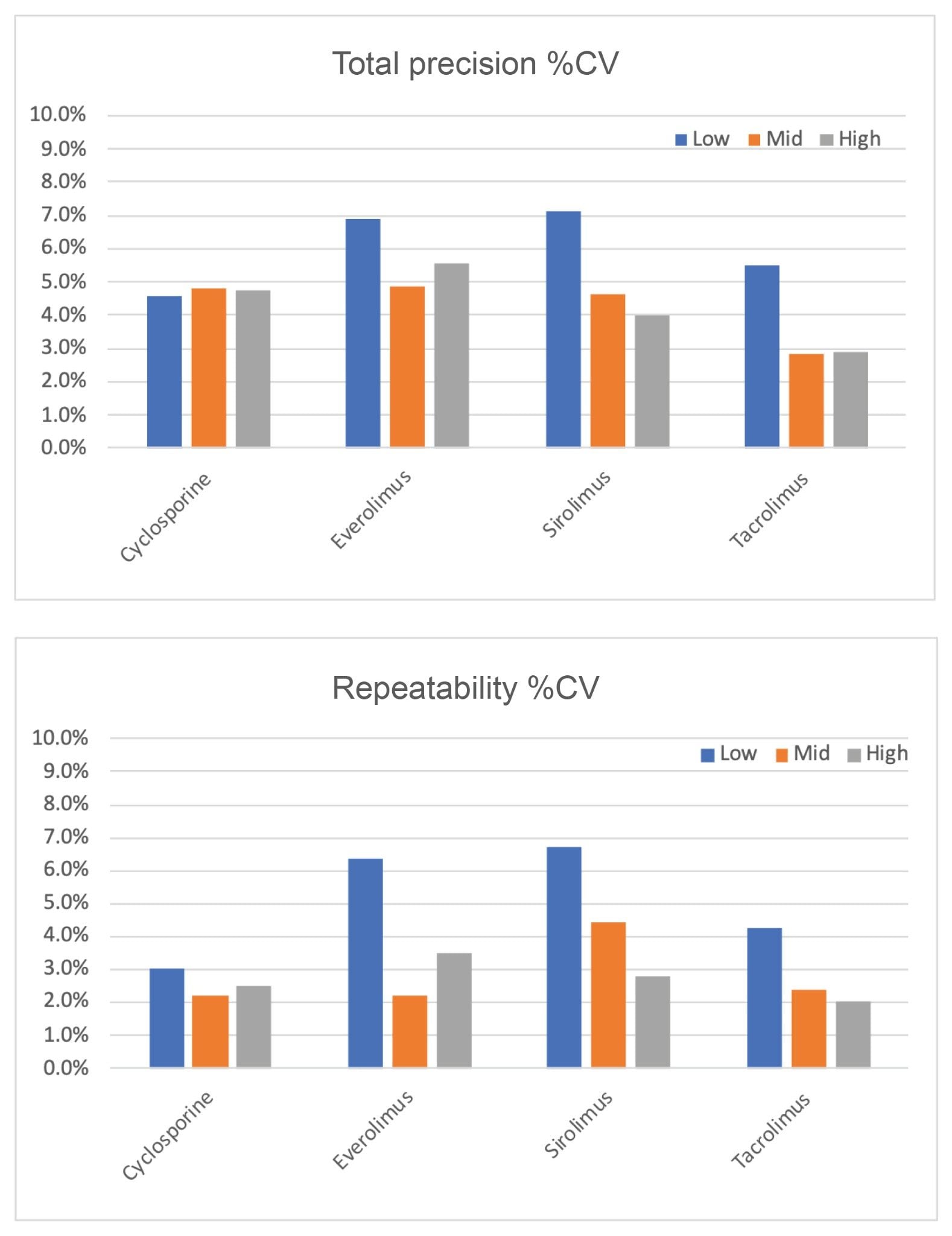
Total precision and repeatability were determined by extracting and quantifying five replicates of three level QC materials per day over five separate days (n=25). Low, mid, and high concentrations were 154.6, 391.6, and 888.2 ng/mL for cyclosporine; 2.2, 8.4, and 22.6 ng/mL for everolimus; 1.9, 7.3, and 19.4 ng/mL for sirolimus and 2.2, 8.4, and 22.8 ng/mL for tacrolimus. Total precision and repeatability were determined to be ≤7.1% CV across all immunosuppressant drugs at the three QC concentrations (Figure 3).
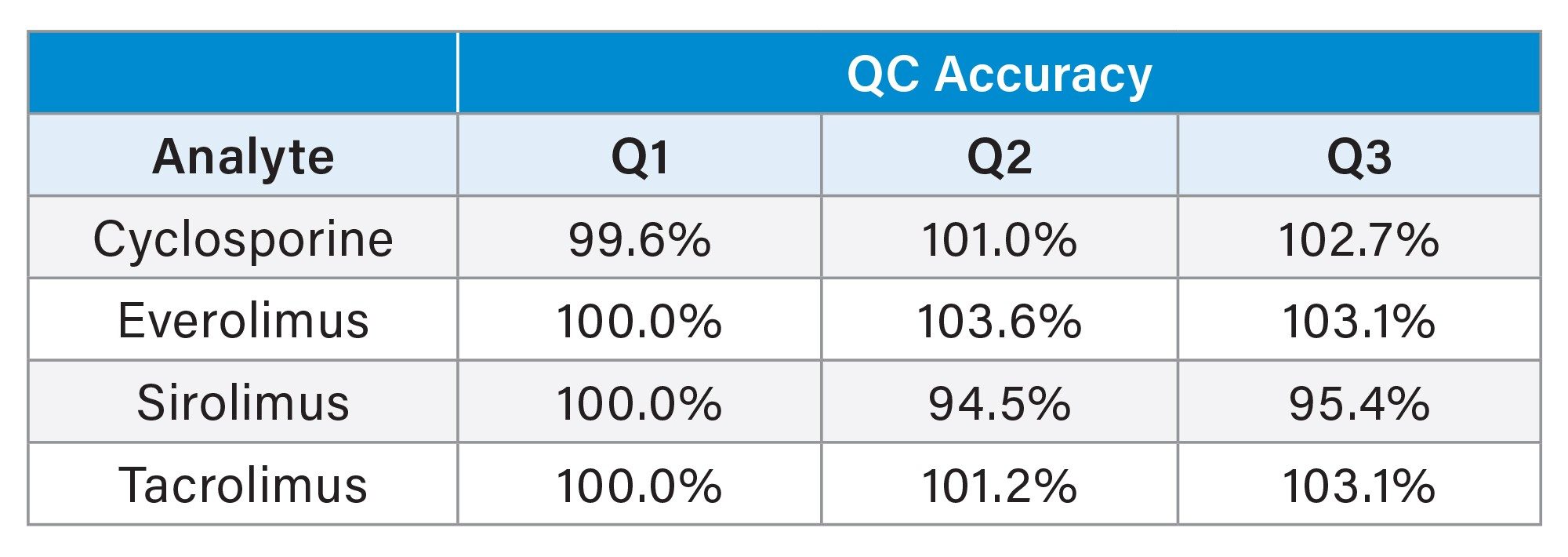
In addition, the accuracy of the QCs was evaluated in comparison to the calibrators over the five analytical; runs. The mean accuracy for the QCs across the four immunosuppressant drugs ranged from 94.5–103.6% (Table 3).
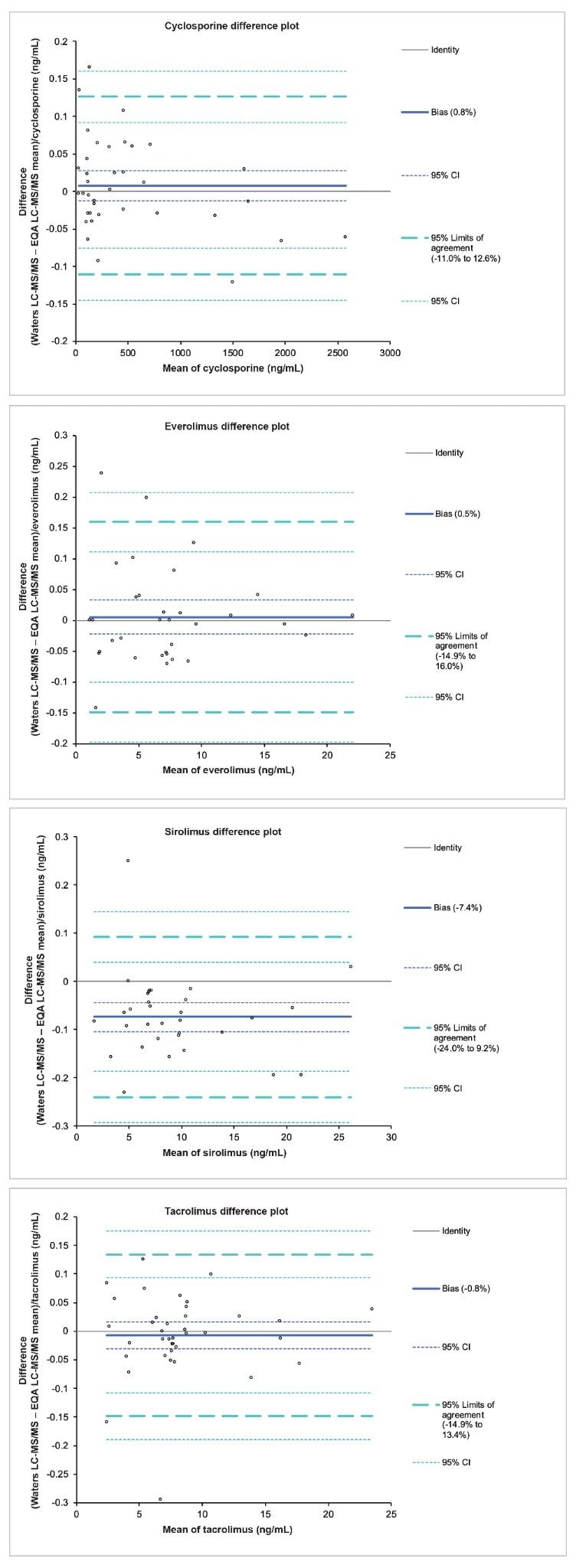
Accuracy was assessed for the four immunosuppressant drugs through the analysis of EQA samples from UK NEQAS. The data obtained was compared to the mass spectrometry method mean for the samples and Altman-Bland agreement was performed on the data sets. Altman-Bland agreement for cyclosporine, everolimus, sirolimus and tacrolimus provided a mean method bias within ±7.4%, demonstrating excellent agreement with the EQA method values for the immunosuppressant drugs (Figures 4a-d).
Conclusion
Through this proof of performance evaluation, it has been demonstrated the MassTrak Immunosuppressant Calibrator and Quality Control Sets (IVD) can provide precise and accurate quantification of the four immunosuppressive drugs in whole blood.
The ACQUITY UPLC I-Class FL with Xevo TQ-S micro Triple Quadrupole Mass Spectrometer is able to provide sufficient analytical sensitivity to analyse lowest required concentrations using only 50 µL sample volume. Excellent levels of precision across the calibration range have been demonstrated with total precision and reproducibility ≤7.1% CV. In addition, the accuracy of the QC set was established with accuracies ranging from 94.5–103.6%. An indication of metrological traceability through agreement to EQA samples was also shown, with the method providing excellent agreement to EQA samples, with mean method bias ±7.4% compared to method mean values from the schemes.
Disclaimer
This method is an example of an application using the instrumentation, software and consumables described in this document. This method has not been cleared by any regulatory entity for diagnostic purposes. The end user is responsible for completion of the method development and validation. MassTrak Immunosuppressant Calibrator and Quality Control Sets are not available for sale in all countries. For information on availability, please contact your local sales representative.
720007582, March 2022