For forensic toxicology use only.
This application demonstrated effectiveness for the analysis of ketamine and xylazine in bone extracts using a captive versus a passive extraction. The automated and fast method development capability of the ACQUITY UPLC with 2D Technology showed the feasibility for the analysis of ketamine and xylazine in rat bones.
While ketamine is traditionally administered for anesthesia or pain management, illicit usage is often seen in forensic cases, either as a recreational drug or as a tool in drug-facilitated sexual assault. Due to difficulties in timely investigation of these incidents, these compounds may no longer be present in samples collected in a standard sexual assault kit, such as blood and urine. Xylazine is an anesthetic agent used in veterinary medicine and does not have FDA approval for use in humans. However, it has recently been observed as a cutting agent in heroin. Post-mortem specimens present many challenges when it comes to toxicological analysis. Due to compound degradation and decomposition factors, analytes present at trace levels may be missed in blood and urine. Hair, bone, and insects have recently been investigated as alternative matrices for analysis due to their increased durability compared to more traditional matrices. However, this durability increases the difficulties in extracting and isolating compounds of interest from these matrices via traditional extraction and chromatography methods. These methods require lengthy extraction times and extensive cleanup steps to obtain samples suitable for analysis. Utilizing multiple instrumentation combinations, analysts can detect compounds at trace levels. Using multidimensional chromatography, several time-consuming extraction steps can be eliminated while still retaining the ability of trace level detection and quantitation.
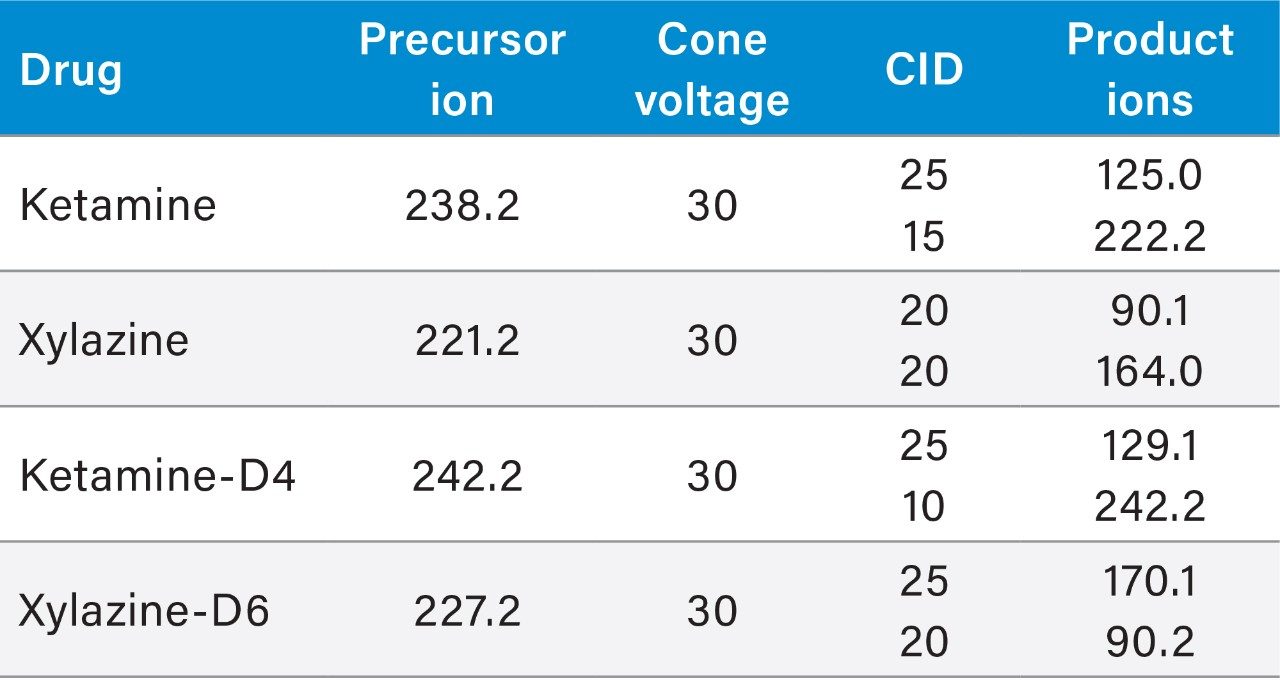
Two MRM transitions, quantification and confirmation, for ketamine and xylazine were selected and optimized. The MRM conditions are listed in Table 1.
For this application, finding the optimum extraction and chromatographic condition for this multi-residue analysis posed a significant challenge. The chromatographic conditions were tested on several trapping chemistries (Oasis HLB, XBridge C18, and XBridge C8) and separation chemistries (BEH C18). The loading (low pH, high pH, and neutral pH) and eluting mobile phase (MeOH + 0.5% formic acid and ACN + 0.5% formic acid) were also optimized using an automated 6 × 6 process.
Bones samples were taken from 10 rats, following xylazine and ketamine administration prior to being euthanized. After homogenization,1 the homogenate was centrifuged at 4000 rpm for 5 minute and the supernatant collected and dilute with MilliQ water for the captive or passive extraction process. The extraction process was performed on pre-conditioned reversed-phase sorbent Oasis HLB SPE Cartridge, 6 cc, 150 mg, or reversed-phase Oasis PRiME HLB SPE Cartridge, 60 cc, 200 mg.
|
Column: |
Oasis HLB Direct Connect HP, 2.1 × 30 mm, 20 μm (p/n 186005231) |
|
Loading: |
At-column dilution 5% (0.1 mL/min loading pump and 2 mL/min diluting pump) |
|
Flow rate: |
2 mL/min |
|
UPLC system: |
ACQUITY UPLC with 2D Technology configured for “Trap & Elute” with At-column dilution |
|
Run time: |
10 min |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 × 50 mm (p/n 176000863) |
|
Column temp.: |
60 °C |
|
Mobile phase A: |
Water + 0.5% formic acid |
|
Mobile phase B: |
Acetonitrile + 0.5% formic acid |
|
Elution: |
5-minute linear gradient from 5% (B) to 95% (B) |
|
Flow rate: |
0.500 mL/min (elution pump) |
|
Injection volume: |
100 μL |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
90.0 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
1100 L/hr |
|
Cone gas: |
50 L/hr |
A successful application starts the optimization process from the detection, moving into separation and finishing with sample preparation. This sequence ensures optimum detection and separation conditions before investing time in sample preparation. The overall analytical process begins with the choice of separation and detection, in most cases a single separation dimension is connected to either a universal or specific detector with software control. In practical terms, liquid or gas chromatography connected to a mass spectrometer is the most popular tool in a wide range of field of activities. Furthermore, since a vast majority of target analytes are water soluble, a single reversed-phase separation (1D) will yield satisfactory results. However, when confronted with a highly complex matrix, the peak capacity of a single resolving dimension does not have enough separation power to separate with baseline resolution, all entities present in a final sample preparation extract. As such the use of multidimensional separation workflow offers performance not available with single dimension workflow. Various configurations can be setup to achieve a specific workflow. In this application, a 2D LC with at-column dilution will be used for the analysis of ketamine and xylazine in rat bones matrix.
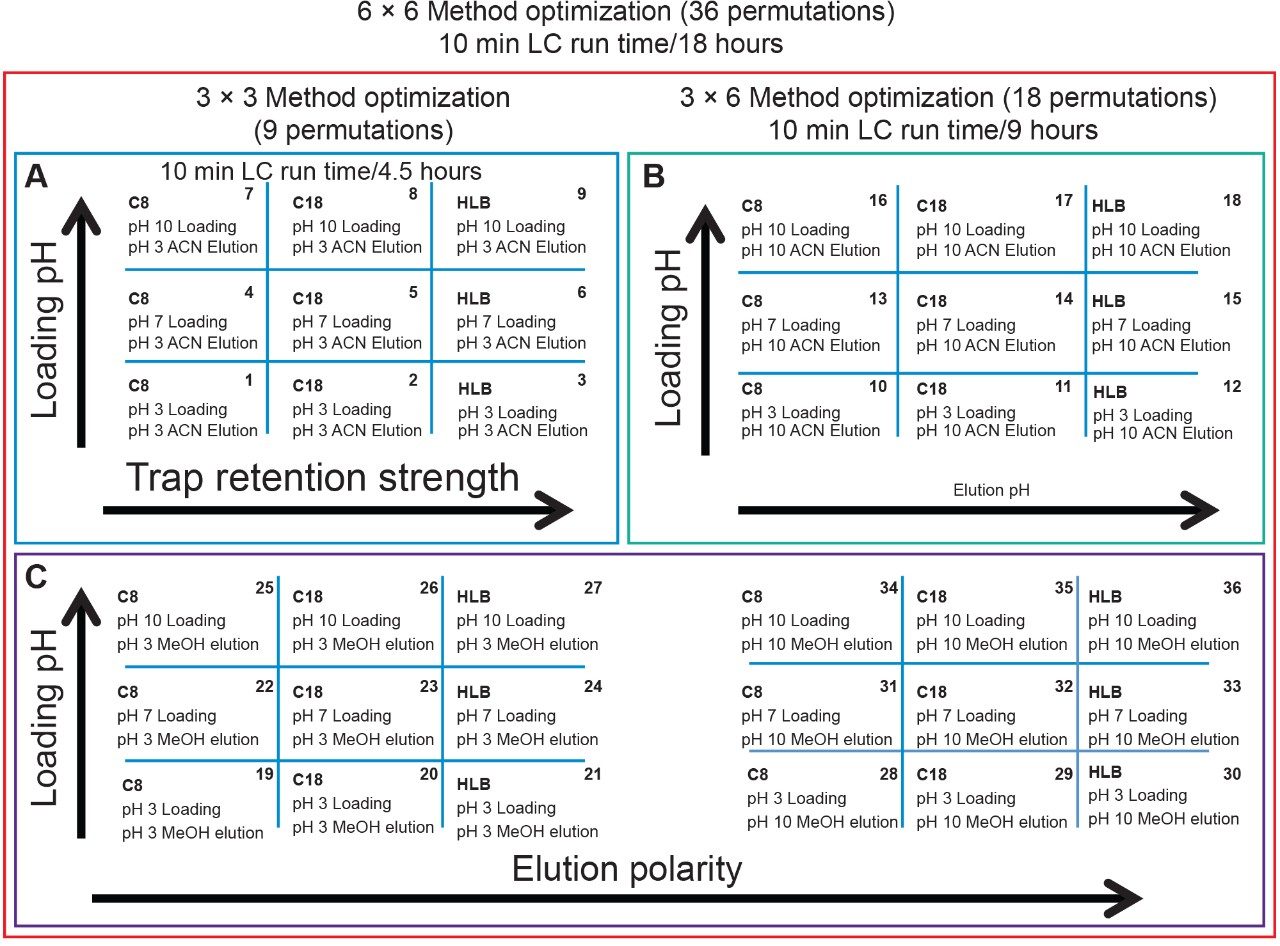
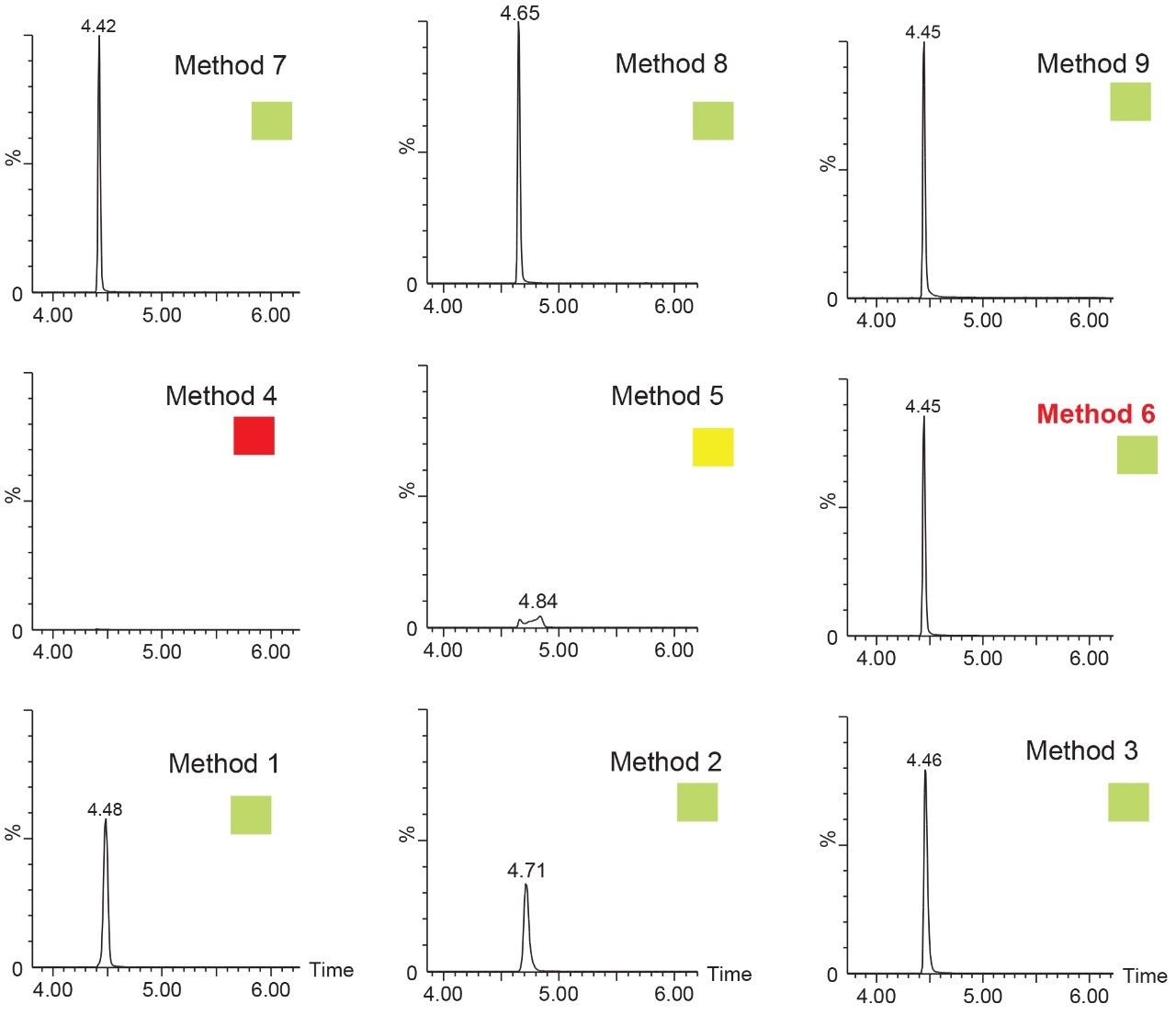
Since the hardware and workflow2-4 aspect is well described in part I of this application note series titled “Novel Extraction Techniques with ACQUITY UPLC with 2D Technology: Part I Pesticides Screening in Drinking Water” (720006588EN), the workflow for this application note will begin with the 6 × 6 grid results for the two-target analyte and displayed in Figure 1A. The grid utilizes a color code to identify which conditions will produce a Gaussian peak shape (green) for quantification, any type of peak distortion (yellow) in combination with green results could be used screening application and last red indicates no signal due either breakthrough effect during loading or poor solubility during elution (See Figure 2A). For this application, ketamine and xylazine show Gaussian peak shape with 16 methods out of 36 permutations. The numerical indicator relates to the peak intensity and is used to select a single or multiple methods for the best sensitivity. In this application, method 6 was selected which utilizes a neutral pH loading onto an Oasis HLB cartridge with a low pH elution with acetonitrile. Since several methods can produce similar results, the selection of method 6 was based on overall cost, which requires no additives in the loading mobile phase. The chromatograms for methods 1 to 9 for ketamine in water are shown in Figure 2.
In all field of activity using either a gas or liquid separation, it will be inevitable that a big portion of the method optimization will be spent on the time-consuming sample preparation. This process will target a wide range of sample matrices with various degrees of complexity. The term “complexity” refers to the amount and diversity of material in the immediate surroundings of a target analyte and in most applications creates high levels of interferences, which during the qualification or quantification will lead to signal distortion and poor analytical performance for the overall application. This is the main reason for spending time on sample cleanup protocols, and also to understand the nature of sample matrix. Several examples can be utilized to describe a low, intermediate, and high complex sample matrix. A low matrix (class A) refers to the ease of isolating a target analyte from a sample. As an example of low matrix sample, pesticides analysis in drinking water can be used to illustrate the concept. In this situation, the target analyte is surrounded by water molecules. From a theoretical point of view, this sample only has two entities, thus the isolation of the target analyte is as simple as removing all water molecules. Several extraction techniques can be used to achieve that objective, of which liquid-liquid extraction (LLE) and solid-phase extraction (SPE) are the most popular ones. Foremost, extraction protocols or practices have not changed in the last one hundred years, all current extraction techniques are derived from either a liquid-liquid or liquid-solid interaction. From this position, when moving into intermediate (class B or opaque liquid sample) and high complexity (class C or solid sample) sample matrix, as the level of interferences increases a single extraction step (i.e., load and elute) that gave excellent results for a class A sample will not yield satisfactory result for a class C. As an example, a class B sample in the pesticides analysis in drinking water would be a surface or ground sample and all the way up to a waste sample for a class C type. Overall, as the complexity increases, a simple load and elute approach should be upgrade with several cleanup steps before final elution. The next section of this application will depict two extraction concepts captive vs. passive extraction for the analysis of xylazine and ketamine in bones extracts.
Once the LC and MS optimization phase were completed, the next step focused on the extraction and sample cleanup of the application. In this instance, since the target matrix is very high on the complexity scale (class C matrix), the extraction protocol requires a robust cleanup methodology and an evaluation of optimum extraction conditions after the homogenization process.
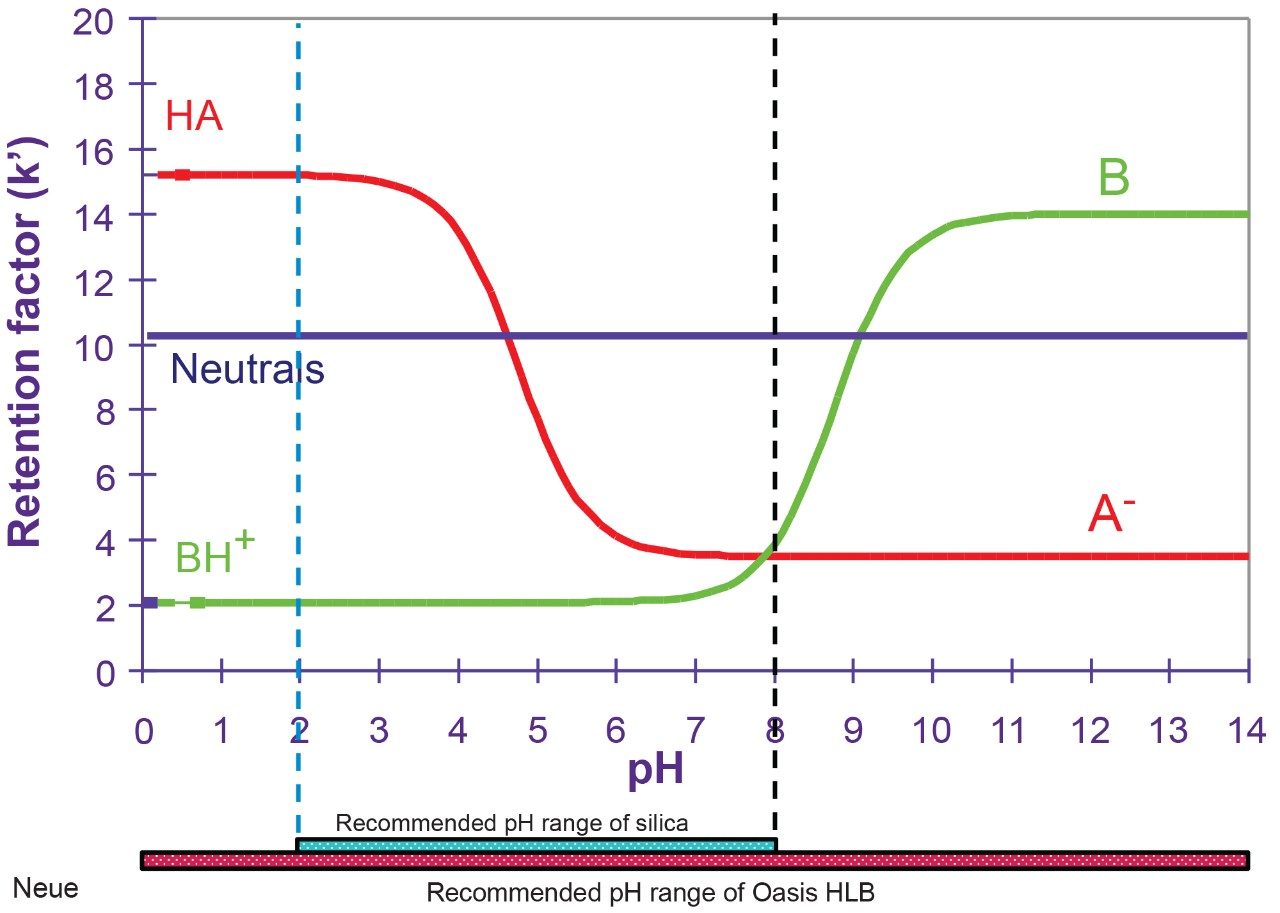
In this application, the first extraction technique we evaluated was the captive extraction approach. The technique, as the name states, utilizes a sorbent that will capture the target analyte during the loading phase. The sorbent could be any type of reversed-phase, HILIC, ion-exchange, or affinity sorbent as the main retention mechanism. For this work, we opted for a reversed-phase sorbent. The next phase of the extraction process targets the removal interferences while keeping the target analyte safely bound to the sorbent, thus avoiding a breakthrough effect and potential low recovery values for the target analyte. In order to isolate a target analyte from a highly complex sample, understanding its retention profile on a given sorbent can lead to utilize various elution parameters (i.e. pH, polarity, solubility, etc.) to create a narrow elution profile for the target analyte and with the least amount of co-eluting interference. Since the introduction of polymer base extraction sorbent with a wide pH range than widely use silica-ligand sorbent, the pH extension capability will lead to an elution profile. In Figure 3, the retention behavior of target analyte with an acidic moitie (i.e., carboxylic acid) versus a basic moitie (primary amine) shows to distinct k' elution profile as pH is varied from 1 to 14. This retention behavior can be used to optimize cleanup steps. In practical, by combining pH and polarity, a complete elution profile can be traced and key elution conditions can be identified for effective cleanup and elution steps. In Figure 4, the elution profile of ketamine was traced using acetonitrile as elution solvent at pH 3 (Figure 4A), next acetonitrile at pH 10 (Figure 4B) and finally using methanol at pH 2 and 10 (Figure 4C).
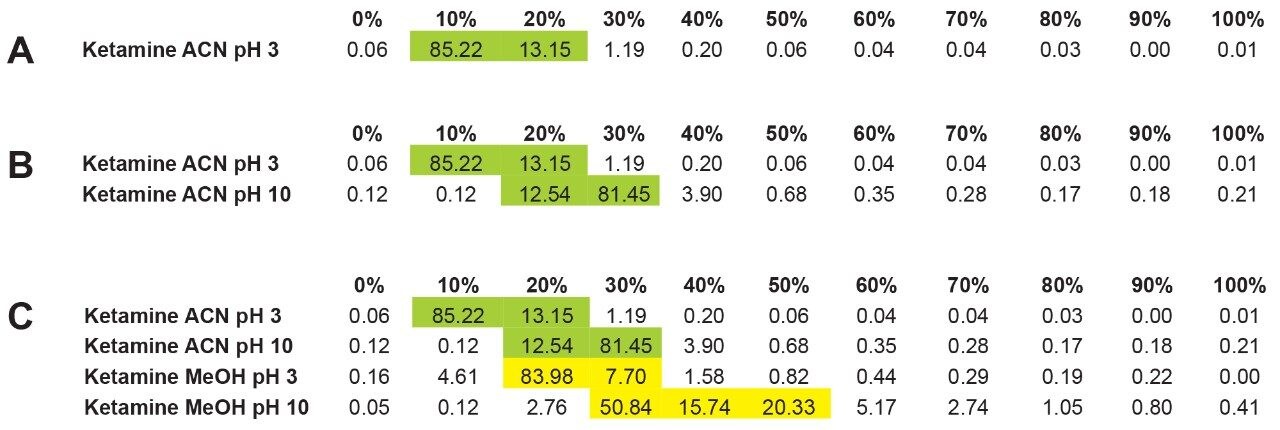
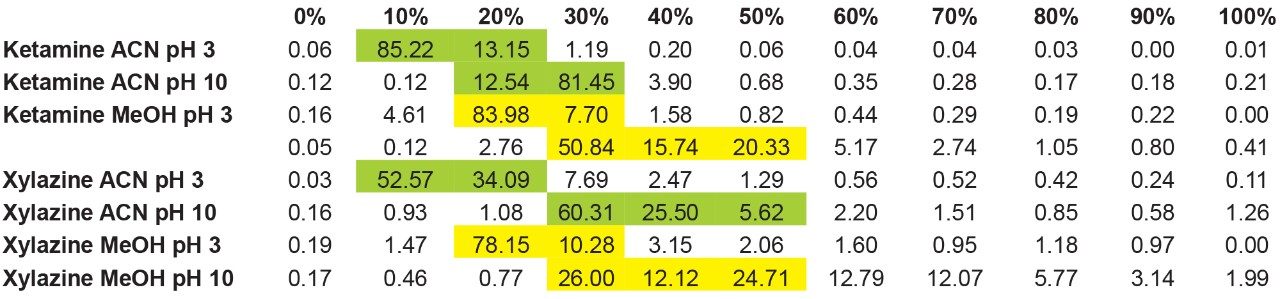
The experimental workflow in Figure 4A shows the retention factor (k') of ketamine with acetonitrile at pH 3 using an incremental percentage of solvent from 0% to 100%. As seen, ketamine shows a very low k' with a 98% elution between 10 and 20% acetonitrile at pH 3. The next step is to look at ketamine elution profile at a higher pH, in this instance pH 10. The pH values used in this work targets the bottom and top end of a pH gradient effect, thus giving clues as the analyte’s pKa value. In this application, ketamine has a dissociation constant of 7.5, meaning at pH 3 ketamine will be in a protonated form and at pH 10 in a neutral form. Under protonated or ionized form, a target of interest will elute off a sorbent in low percentage of organic solvent. In neutral form, the elution will require high percentage as seen in Figure 4B, ketamine eluted in a slightly higher elution bracket at 20 to 30% acetonitrile at pH 10. This elution behavior corresponds well to theory regarding a molecule containing a basic moitie. Next, by substituting the elution solvent polarity, in this instance acetonitrile to methanol, the results showed, in Figure 4C, that ketamine eluted with methanol at pH 3 is in the same range as acetonitrile at pH 10. A fact worthy of pointing out, 83% of ketamine eluted at 20% methanol pH 3, but with methanol pH 10, ketamine is spread out between 30 to 50%.
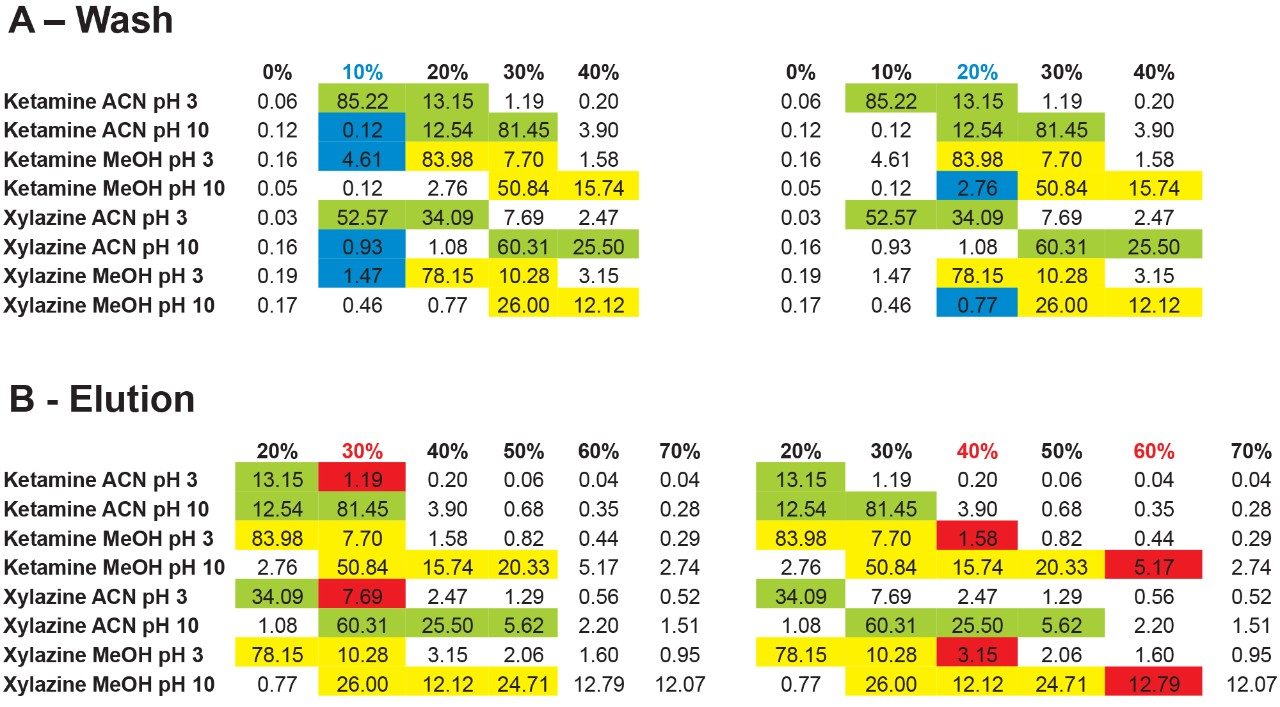
The results showed that ketamine shows a net preference for acetonitrile in term of solubility. By adding the overall results for xylazine, the next phase is to identify a cleanup and elution sequence for both target analytes, as shown in Figure 5. The results demonstrate that both analytes are showing similar elution profiles. For this application, three potential wash conditions highlighted in blue can be set at 10% acetonitrile pH 3, 10% acetonitrile at pH 10 and 20% methanol at pH 10 without any major breakthrough for both analytes (see Figure 6A). As for the elution conditions (see Figure 6B), reducing the organic percentage gap between wash and elution will ensure a narrow hear cutting effect thus reducing matrix effect when compared to a 100% organic elution. The elution is showing three prospect conditions highlighted in red at 30% acetonitrile pH 3, 40% methanol at pH 3, and 60% methanol at pH 10. By adding more data points to this experiment, it would bring a tighter range between the wash and elution step. For this application, we opted to proceed with a 20% methanol at pH 10 for the wash step and using a 40% methanol at pH 3 for elution.
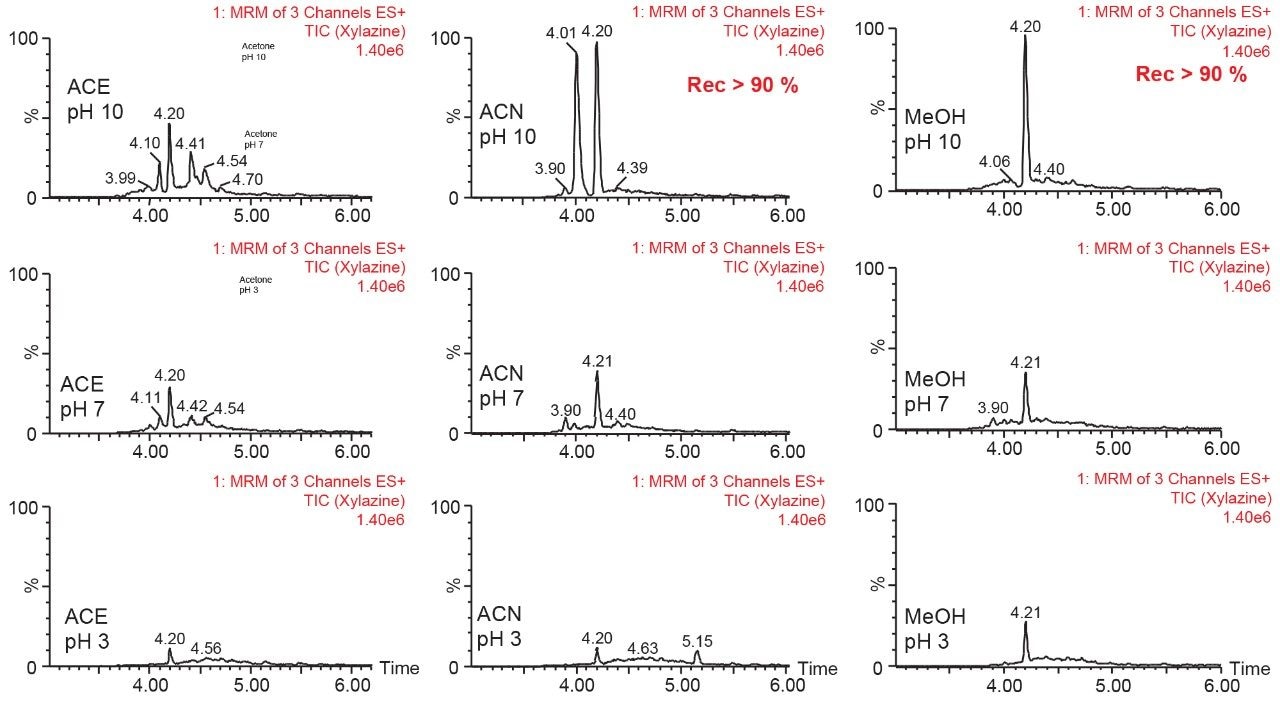
The final phase of this application was to identify the proper extraction solvents conditions for a solid-liquid extraction with rat bones matrix. Following conditions from previous work on the topic, three solvents (methanol, acetonitrile, and acetone) and three pH values (3, 7, and 10) were selected to evaluate which conditions will yield the best recovery (See Figure 7). The methanol and acetonitrile at pH 10 extracts gave recovery values above 90% for both analytes, with the methanol pH 10 producing a single target peak with a slightly raised background signal, potentially indicating co-extraction of minor interferences.
The previous section described a typical captive workflow when using a reversed-phase sorbent in a solid-phase extraction format (either barrel or 96-well plate). The workflow largely focuses on optimizing the load, wash, and elution steps as separate entities, which can be quite laborious and time consuming.
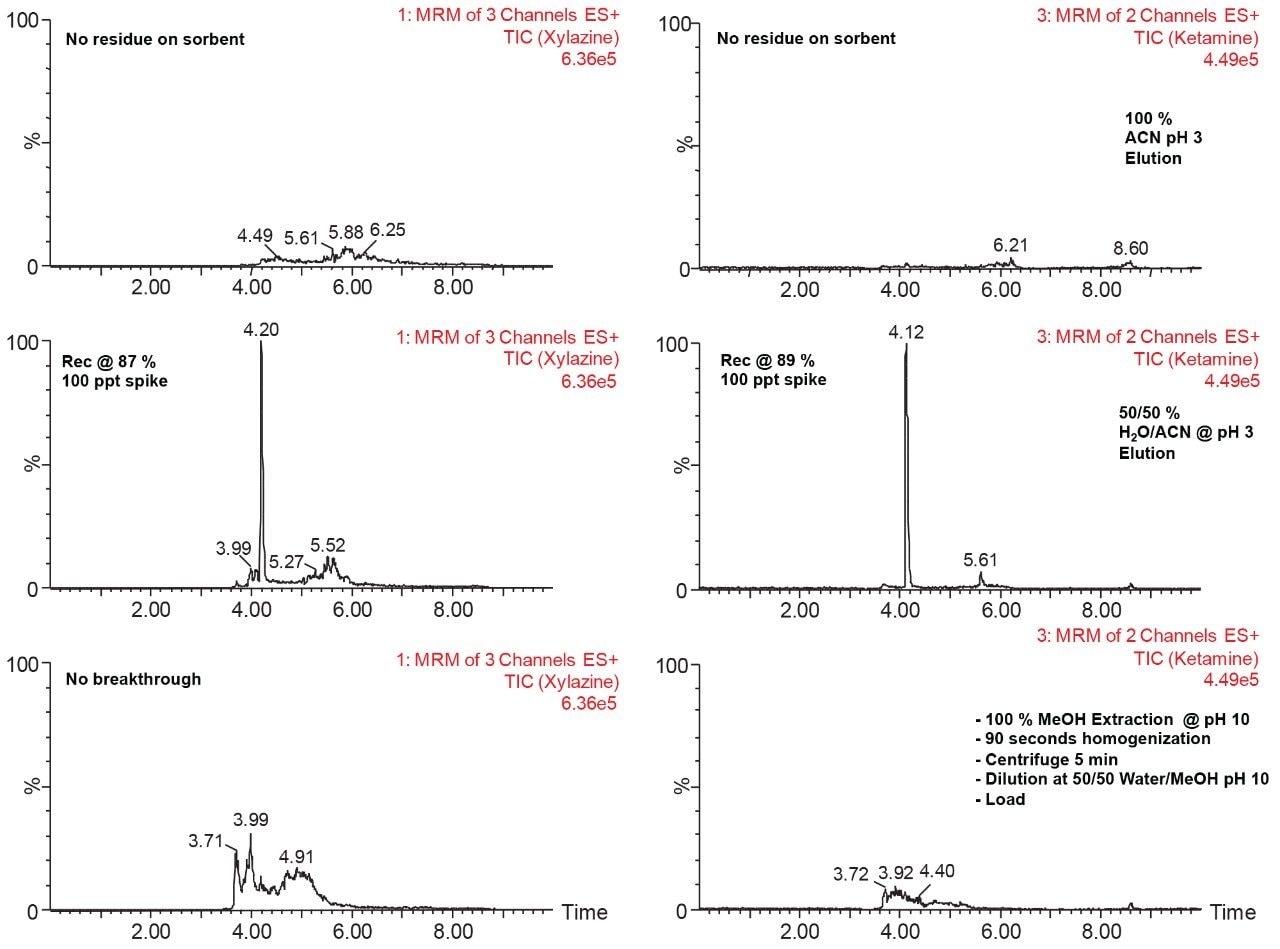
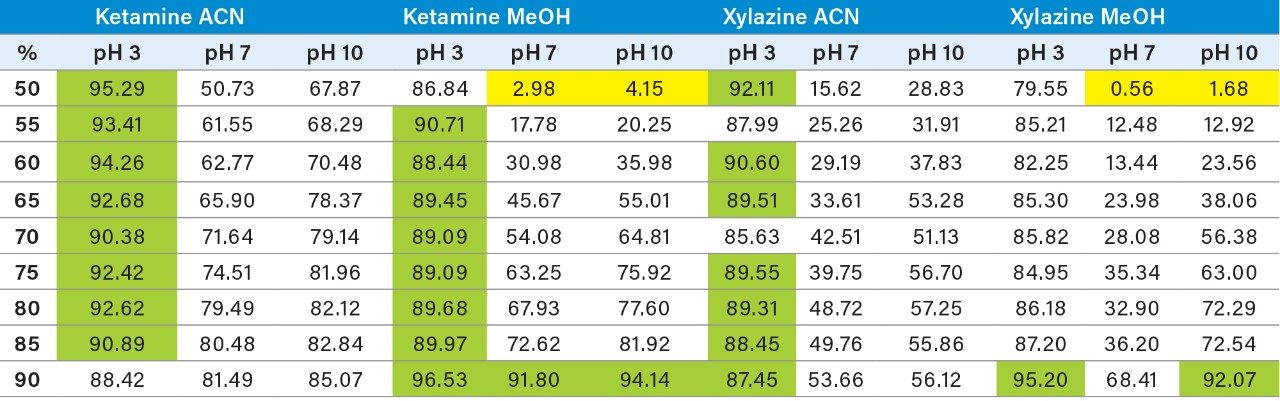
With a passive extraction process, a sorbent SPE bed is blended with additional particles (polymer and/or silica based), thus offering a wider range retention mechanism. The strategy behind a blended SPE offers the option to create a single step extraction, meaning that the loading and elution phase of the extraction are combined to create an intentional breakthrough effect of the target analyte during loading, and at the same capturing mid to late eluters on the sorbent. The major difference relates to the composition of the sample before the loading step. With a captive SPE approach, the target analyte is loaded onto the sorbent bed in 100% aqueous medium or with an organic solvent up to 5%. The organic value is low enough to ensure total capture during loading phase. For a passive extraction, the organic percentage is purposely increased to ensure a total breakthrough of the target analyte but with a minimum level of organic to ensure capture of late eluting interferences. As an example, Figure 8 shows recovery values for ketamine and xylazine with two organic solvents, at three pH values with increasing percentage from 50% to 100%. Overall, the main objective for a passive extraction is to produce a breakthrough effect over 90% which in fact would produce the same value for recovery value for a captive extraction. In this instance, high pH value appears to affect the breakthrough recoveries with lower values than at low pH for both acetonitrile and methanol. A surprising effect occurred during evaluation, with a pH 7 and 10 values at 50% acetonitrile or methanol, both ketamine and xylazine showed less than 5% breakthrough effect (yellow) in comparison to 80% in methanol at pH 3 or 90% in acetonitrile at pH 3. Clearly, k' is playing a key role during the loading step. This phenomenon was quickly identified to combine this retention behavior with the solid-liquid extraction of bone sample homogenization step. With a sample extract already in 100% organic solvent (after centrifugation solid particulates are in a pellet with a top supernatant layer), a simple aqueous dilution can adjust the water/organic ratio for immediate loading on a passive extraction. With captive extraction, the organic extract would require a solvent exchange to aqueous to be compatible the loading step for the extraction process. In Figure 8, the bottom chromatograms show the results for xylazine and ketamine with a 50/50 water/MeOH at pH 10. As expected, both analytes are safely retained during the loading step which could be perceived as count intuitive as explained earlier. However, by eluting with a 50/50 acetonitrile at pH 3, the middle chromatograms are showing Gaussian peak shape for both analytes with recoveries close to 90%. The interesting result of this workflow is the relatively low abundance of interference from a high complex sample matrix. As a second fact, since both the loading and eluting phase utilize the same water/organic, adjusting the pH and exchanging the solvent polarity, the results are comparable to a captive extraction protocol. The top chromatograms were eluted at 100% acetonitrile at pH 3 to see if there any analyte residue left on sorbent. In this instance, the 10% could be lost during the loading phase or a matrix effect.
This application demonstrated effectiveness for the analysis of ketamine and xylazine in bone extracts using a captive versus a passive extraction. The automated and fast method development capability of the ACQUITY UPLC with 2D Technology showed the feasibility for the analysis of ketamine and xylazine in rat bones. The quantification limit was set at 100 ppt using a 1 g of sample. The micro extraction protocol offered the option to evaluate several elution parameters in a short time period. The 2D LC results were analyzed using an over-night run multi-methods sample list (24 hours). With the extraction protocol optimized, the final protocol produced a clean extract in 30 minutes without any evaporation to dryness and reconstitution into initial mobile-phase conditions. The captive and passive extraction protocols gave a 90% recovery average for both drugs.
720006587, July 2019