
This is an Application Brief and does not contain a detailed Experimental section.
In this application brief, we demonstrated that SPE is a better clean up technique for difficult semisolids like hydrocortisone valerate ointment compared to the original USP solvent extraction procedure.
Liquid chromatography (LC) assays of formulated pharmaceuticals generally require sample preparation to provide a sample solution that is free of interferences which can adversely affect the measurement. Sample preparation for semisolids can be particularly difficult due to the excipients present in the formulations. A common clean up procedure for such samples is solvent extraction, in which a diluent is used to dissolve the pharmaceutical components. The solution is then centrifuged and/or filtered to remove insoluble materials prior to analysis. However, these simple separation steps may be insufficient to completely remove the excipients. In such cases, solid-phase extraction (SPE) can be helpful, since SPE is a more selective and efficient separation process.
In this application brief, we demonstrate the use of SPE to prepare a hydrocortisone valerate ointment for LC analysis. Hydrocortisone is a medium potency corticosteroid used to provide relief from itching and inflammation due to various dermatologic conditions. This semisolid ointment is an “oil-in-water” type emulsion, containing excipients such as carbomer homopolymer and petrolatum.
The USP monograph for hydrocortisone valerate1 specifies sample preparation using a hot solvent extraction with methanol/water (3:1, v/v) at “steam bath” temperatures for ca. 30 seconds. The extract is cooled to room temperature and ethyl benzoate is added as an internal standard (IS). The solution is centrifuged for 5 minutes and then filtered. This filtrate is the analysis solution. When this solution is placed in the refrigerator for storage or in a cooled LC autosampler for analysis (e.g. below 10 °C), white agglomerates form, as pictured in Figure 1. This result indicates that the excipients are not sufficiently removed, which can lead to injector and column clogging over time.

Solid-phase extraction is a sample preparation technique where partitioning occurs between a solid and liquid phase. It produces clean analyte solutions from complex samples for quantitative analysis. Given the above observation, two different SPE sorbents were examined to more completely remove the excipients from hydrocortisone valerate ointment samples.
The first SPE procedure utilized a Sep-Pak Silica Cartridge. The Sep-Pak Silica 1 cc, 50 mg Cartridge (p/n: WAT054980) was conditioned with hexane/ethyl acetate (70:30, v/v). A hot solvent extraction of the hydrocortisone valerate ointment was performed using this solvent mixture (at ca. 55 °C for about 30 seconds). After cooling, the extract was loaded onto the cartridge. The sorbent bed was washed with the same solvent mixture, and then elution proceeded using a methanol/water solution (3:1, v/v). The eluate was then spiked with the above mentioned internal standard of ethyl benzoate and analyzed using LC.
The second SPE procedure employed a newer SPE sorbent, Oasis PRiME HLB. The Oasis PRiME HLB 1 cc, 30 mg Cartridge (p/n: 186008055) was loaded with the supernatant (containing the above mentioned internal standard) of the solvent extract from the USP method. The load effluent solution was then analyzed using LC.
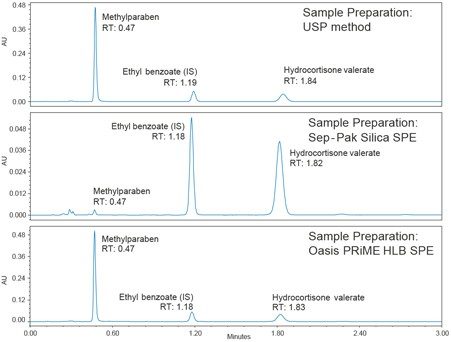
When the analysis solutions prepared using SPE were subjected to the same 10 °C conditions, there were no white agglomerates (see Figure 1). This indicates that SPE is a better clean up technique compared to the original USP procedure. The recovery with both SPE methods was within the range specified in the USP method (90% to 110%). Figure 2 depicts chromatograms obtained from the analysis solutions prepared via the USP method clean up and the Sep-Pak Silica and Oasis PRiME HLB SPE techniques. An ACQUITY Arc System with a 2998 PDA Detector was used, with an XSelect HSS C18 SB XP, 2.5 µm, 3.0 x 75 mm Column (p/n: 186006166). The chromatographic conditions were scaled from the original compendial USP LC method.
Filtration and centrifugation are both techniques that separate based on physical properties. Filtration uses a sieving mechanism to separate by size, whereas centrifugation utilizes centrifugal force to separate by density. Depending on the sample, centrifugation and filtration may be insufficient to separate analytes from the excipients. Solid-phase extraction exploits the differences in chemical properties of the analytes and excipients. The difference in affinities with the sorbent provides a powerful mechanism for such separations. Two different SPE sample clean up mechanisms were investigated in this technical note.
Sep-Pak Silica is composed of a polar, silica sorbent bed which retains more polar analytes. In our case, the less polar excipients pass through in the load and wash steps. Switching to the polar elution solvent releases the analytes in the elute step. Conversely, with Oasis PRiME HLB, the less polar sorbent bed retains the hydrophobic excipients and the more polar analytes pass through. Even though both SPE methods are capable of removing the excipients from the hydrocortisone valerate ointment, there are advantages of the Oasis PRiME HLB protocol over the Sep-Pak Silica method.
Sample solutions must be free of excipients that can affect the analysis, decrease column life, and damage the instrument. In this note, we demonstrated that SPE is a better clean up technique for difficult semisolids like hydrocortisone valerate ointment compared to the original USP solvent extraction procedure.
720006180, January 2018