This application note describes the fast, sensitive quantification of rituximab from plasma using ProteinWorks Auto-eXpress Digest and SPE Sample Preparation Kits with automated sample preparation performed on the Hamilton Microlab STAR liquid handler.
The ProteinWorks Auto-eXpress Digest and SPE Kits with automated sample preparation using the Hamilton Microlab STAR liquid handler was successfully used to quantify rituximab from a typical set of standard curve and QC samples in rat plasma. Limits of quantification of <0.1 μg/mL was readily achieved, while maintaining excellent linearity and precision. The total sample prep time including digestion and SPE was under 8 hours. This automated and standardized, kit-based approach enables inexperienced users to immediately obtain meaningful data in discovery studies in order to make time sensitive and critical project decisions.
Automated and standardized approach for high throughput protein quantification; broadly applicable optimized digest kit eliminates method development; high sensitivity detection (0.1 µg/mL) for the mAb, rituxan.
In the past decade, monoclonal antibodies (mAbs) have emerged as important therapeutics for the treatment of life-threatening diseases. In fact, mAbs are currently considered the fastest growing class of therapeutics with an estimated market value approaching $138.6 billion by 2024.1
Rituxan (rituximab), is a monoclonal antibody against B-lymphocytes CD20 surface protein used to treat lymphomas, leukemias, transplant rejection, and rheumatoid arthritis.2 Like many other antibody therapeutics, Rituxan finds itself in the middle of patent expiry, with loss of exclusivity in Europe in 2013 and US in 2018.3 This loss of patent exclusivity has resulted in an increase in biosimilar development activity by both biosimilar developers and innovator, putting demand on ‘traditional’ small molecule bioanalytical labs to develop methods for their accurate quantification.

While mass spectrometry (MS) based analytical methodologies offer advantages such as: multiplexing, broad dynamic range, superior selectivity, and short method develop times, analysis of large molecule biologics like rituximab can be very challenging. This is especially true for protein quantification workflows, which often employ complicated sample preparation with affinity purification, enzymatic digestion, peptide level SPE clean-up, and analysis of the resulting peptides. In particular, the digestion workflow encompasses a multitude of sub-segments, each with many steps requiring optimization, making it difficult for scientists to develop sensitive and robust methods for mAb quantification. An automated and standardized approach to protein bioanalysis, which supplies all the necessary reagents and protocols, would enable novice bioanalytical scientists to successfully quantify proteins with ease. This application note describes the fast, sensitive quantification of rituximab from plasma using ProteinWorks Auto-eXpress Digest and SPE Sample Preparation Kits with automated sample preparation performed on the Hamilton Microlab STAR liquid handler. This workflow is highlighted in Figure 1. Using this automated kit-based approach with pre-measured reagents and a universal protocol, an LLOQ of 0.1 µg/mL of rituximab was achieved.
Rituximab spiked rat plasma samples (40 µL) were immunopurified using a 96-well Protein A agarose-based plate (GE Healthcare P/N: 28-9031-33). The post-affinity purified plasma was then digested and peptide-level purification was completed using the ProteinWorks Auto-eXpress Low 5 Digest and µElution SPE Clean-up Kits, respectively. Specifically, 80 µL of purified eluate (equivalent to 28 µL plasma) was digested. Following digestion, 125 µL of the sample was processed by SPE and eluted with 50 µL of elution solution and then diluted with 50 µL of water and mixed. This sample was then subject to LC-MS analysis using the Waters ACQUITY UPLC and Xevo TQ-S Mass Spectrometer. All steps in the digestion and peptide purification workflow were carried out using the Hamilton Microlab STAR liquid handler (STAR) and included ProteinWorks protocols (Figure 2A and 2B). The STAR deck layout is shown in Figure 3.
|
LC system: |
ACQUITY UPLC |
|
Detection: |
Xevo TQ-S Mass Spectrometer, ESI+ |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300Å, 1.7 μm, 2.1 mm x 150 mm |
|
Column temp.: |
55 °C |
|
Sample temp.: |
10 °C |
|
Injection vol.: |
10 μL |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.300 |
98.0 |
2.0 |
6 |
|
1.00 |
0.300 |
98.0 |
2.0 |
6 |
|
7.00 |
0.300 |
60.0 |
40.0 |
6 |
|
8.00 |
0.300 |
10.0 |
90.0 |
6 |
|
8.80 |
0.300 |
10.0 |
90.0 |
6 |
|
9.00 |
0.300 |
98.0 |
2.0 |
6 |
|
10.00 |
0.300 |
98.0 |
2.0 |
6 |
|
Capillary: |
2.9 kV |
|
Source offset: |
60 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
1000 L/Hr |
|
Collision gas flow: |
0.15 mL/Min |
|
Nebulizer gas flow: |
7 Bar |
|
Data management: |
MassLynx (v4.1) |
|
Quantification software: |
TargetLynx |
|
Automation platform: |
Hamilton Microlab STAR liquid handler |
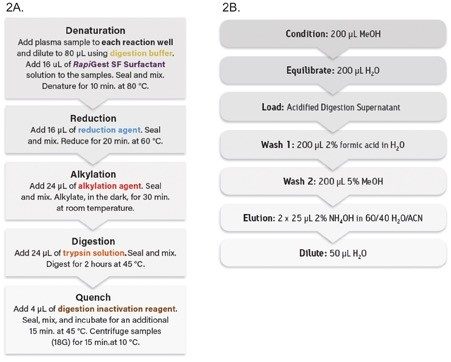
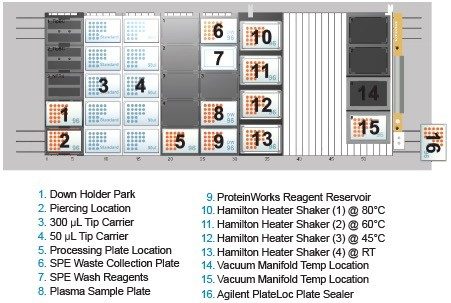
With Rituxan’s US patent expiration date of 2018 drawing near, the focus on this mAb in bioanalytical labs has greatly increased. However, typical workflows for its quantification are complex and time consuming, making the development of a high sensitivity quantification method challenging. While there have been great advances in MS for large molecule detection and quantification, the complex and time consuming sample preparation (e.g., affinity purification, digestion, and SPE) has become a major bottleneck. With the need to simplify these complex workflows and maintain adequate throughput, incorporating an automated, kit-based sample preparation strategy would provide a solution to this bottleneck.
In this application note, we have used the ProteinWorks Auto-eXpress Digest and SPE Kits with automated sample preparation using the Hamilton STAR to simplify sample preparation. Rituximab samples were affinity purified, digested, and peptides extracted using SPE in less than 8 hours, enabling sample analysis to begin the same day. LC-MS/MS quantification of multiple signature peptides was performed using a Xevo TQ-S tandem quadrupole MS. Chromatographic separation was achieved using an ACQUITY UPLC and an ACQUITY UPLC Peptide BEH C18, 300Å, 1.7 µm, 2.1 x 150 mm Column, using a linear gradient with 0.1% formic acid in water and acetonitrile (flow rate 0.3 mL/min) and a sample injection volume of 10 µL. Signature tryptic peptides and MS conditions used for rituximab quantification are summarized in Table 1.
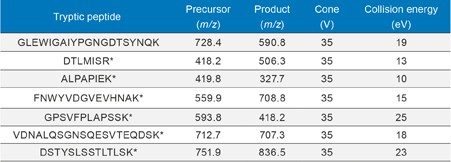
Table 1. MRM conditions for rituximab, including precursor and product ions.
* Indicates generic signature peptide based on Human IgG.
Using the optimized protocol and supplied reagents, executed on the STAR, only 28 µL plasma was needed to achieve a detection limit of <0.1 µg/mL for rituximab. Standard curves were linear up to 3.5 orders of magnitude with the average accuracies for standard curve points between 100–102% for the various signature peptides. Linearity and accuracy of the standard curves for each peptide are summarized in Table 2.
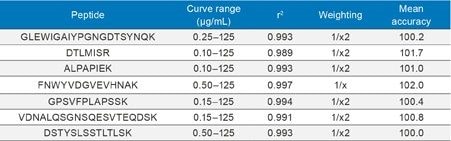
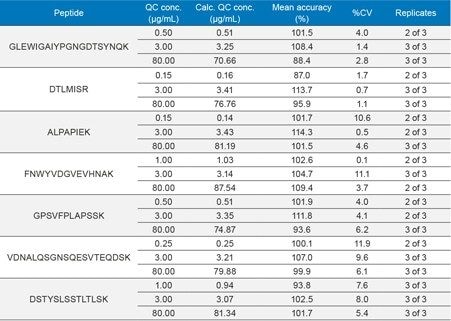
At the same time, QC statistics (summarized in Table 3) easily met regulatory guidelines,4 with average precision values <12%. In fact, the average % CV for QC samples across all of the peptides was <5%.
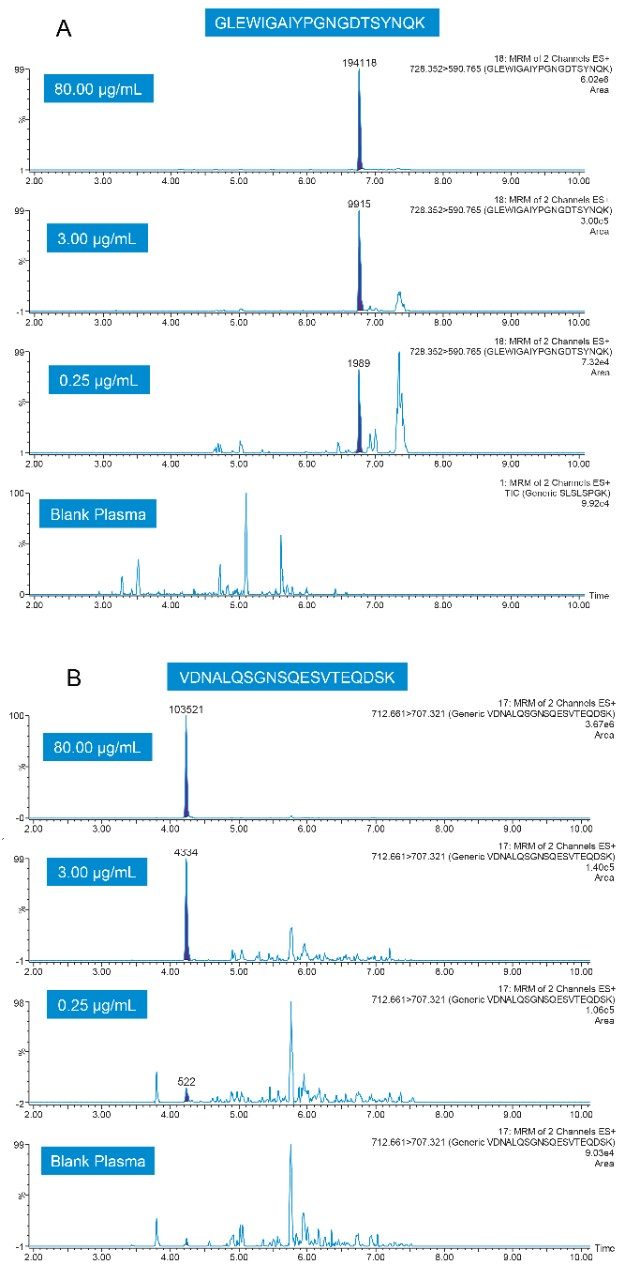
Representative QC chromatograms for the GLEW and VDNA signature peptides, demonstrating the high level of sensitivity achieved with this method, are shown in Figure 4, Panels A and B.
In this study, using a universal digestion protocol and SPE method specifically designed for tryptic peptides, performed on the STAR eliminated the need for discovery-stage large molecule method development. This streamlined sample preparation process, maximizes laboratory productivity, reduces the potential for errors, all while achieving excellent analytical performance.
The ProteinWorks Auto-eXpress Digest and SPE Kits with automated sample preparation using the Hamilton Microlab STAR liquid handler was successfully used to quantify rituximab from a typical set of standard curve and QC samples in rat plasma. Limits of quantification of <0.1 µg/mL was readily achieved, while maintaining excellent linearity and precision. The total sample prep time including digestion and SPE was under 8 hours. This automated and standardized, kit-based approach enables inexperienced users to immediately obtain meaningful data in discovery studies in order to make time sensitive and critical project decisions.
720006165, January 2018