For research use only. Not for use in diagnostic procedures.
In this application, we report the use of the newly developed 150-μm ionKey/MS System for the multiplexed quantitation of five steroid compounds in human serum. The use of the ionKey Source and the 150-μm iKey Separation Device yielded a 100-400 fold increase in on-column sensitivity while at the same time decreasing solvent usage by 150 fold as compared to standard flow methods. The increased on-column sensitivity allowed for simplification of the steroid extraction procedure which in turn streamlined the sample preparation and reduced per/sample assay cost.
A highly analytically sensitive multiplexed assay was developed for targeted quantitation of five steroids in human serum. The use of the ionKey source and the 150 µm iKey Separation Device yielded a 100–400 fold increase in on-column sensitivity while at the same time decreasing solvent usage by 150 fold as compared to standard flow methods. The increased on-column analytical sensitivity allowed for simplification of the steroid extraction procedure which in turn streamlined the sample preparation and reduced per/sample assay cost.
The measurement of steroids in human serum is an important clinical research tool. Traditionally, these assays are performed using a variety of biochemical techniques including radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescent immunoassay (CLIA). However, immunoassays suffer from antibody cross-reactivity with structural isomers which has been shown to result in an overestimation of steroid levels. Recently, LC-MS/MS has emerged as a viable alternative for this important assay in the clinical research setting. While providing improved accuracy as compared to antibody based techniques, standard flow LC-MS/MS assays also consume high levels of solvent and often require time-consuming sample extraction procedures such as liquid-liquid or solid phase extraction to adequate analytical sensitivity.
In this application, we report the use of the newly developed 150 µm ionKey/MS System for the multiplexed quantitation of five important steroid compounds in human serum: testosterone, dihydrotestosterone, progesterone, cortisone, and cortisol. The reduced flow method results in a 150 fold decrease in solvent consumption and a 100–400 fold increase in on-column analytical sensitivity.1
|
LC system: |
nanoACQUITY UPLC |
|
Sample loop: |
5 μL |
|
Column: |
iKey BEH C18 130, 1.7 μm, 150 μm x 50 mm |
|
Column temp.: |
45 °C |
|
Flow rate: |
3.06 μL/min |
|
Mobile phase A: |
Water + 0.1%formic acid |
|
Mobile phase B: |
Methanol + 0.1% formic acid |
|
Volume injected: |
0.5 μL using partial loop mode |
|
Time (min) |
%A |
%B |
Curve |
|---|---|---|---|
|
Initial |
90.0 |
10.0 |
Initial |
|
0.25 |
90.0 |
10.0 |
6 |
|
1.00 |
45.0 |
55.0 |
6 |
|
7.50 |
5.0 |
95.0 |
6 |
|
8.00 |
55.0 |
10.0 |
6 |
|
12.00 |
55.0 |
10.0 |
6 |
|
Mass spectrometer: |
Xevo TQ-S |
|
Acquisition mode: |
MRM |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.2 kV |
|
Source temp.: |
100 °C |
|
Source offset: |
50 V |
|
Collision gas: |
argon |
|
Dwell times for all compounds: |
0.011 s |
Serum samples were precipitated with 3.7 volumes of ice cold methanol containing stable isotope-labeled internal standards for each steroid at a level of 10 ng/mL. Samples were incubated at -80 °C for 30 minutes, centrifuged at 3270 x g for 10 minutes and supernatant was collected. All sample preparation and injections were conducted in 96-well plates. 0.5 µL of extracted serum was injected and separation was performed using a nanoACQUITY UPLC connected to an ionKey source using a 150 µm iKey packed with BEH C18 (1.7 µm particles). The column effluent was monitored using a Xevo TQ-S Mass Spectrometer operated in multiple reaction monitoring (MRM) positive ion electrospray mode.
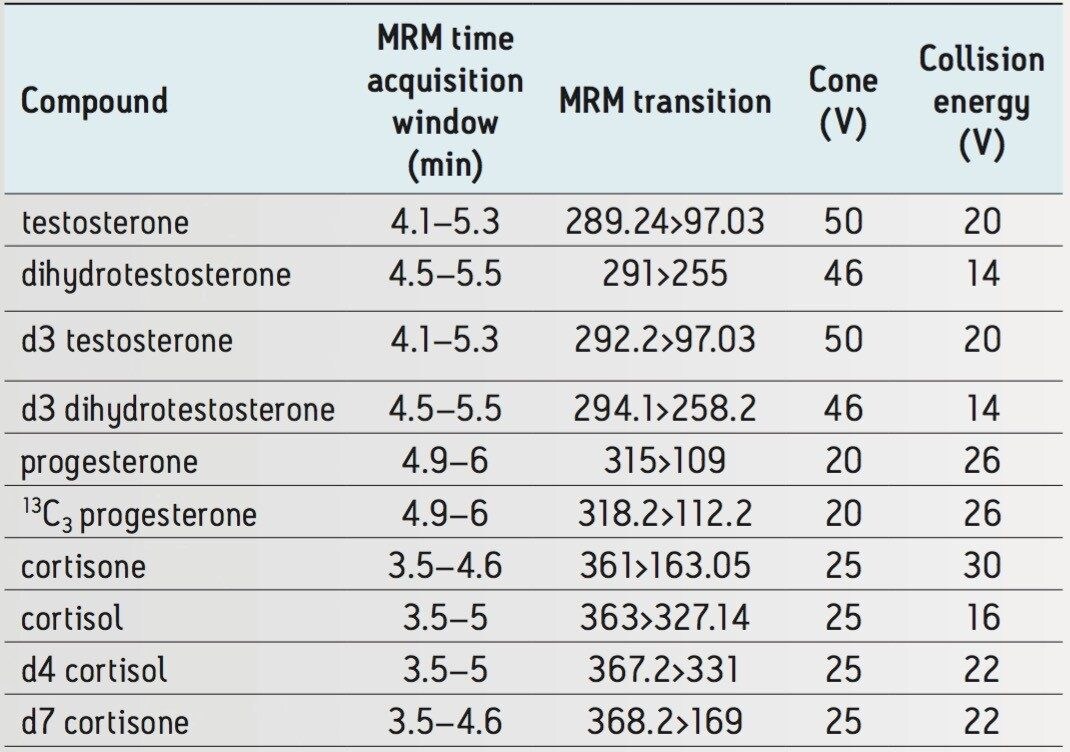
Quantification was performed using linear regression against a standard curve in MassLynx Software. Peak areas for each compound were normalized to the corresponding internal standard in each sample.
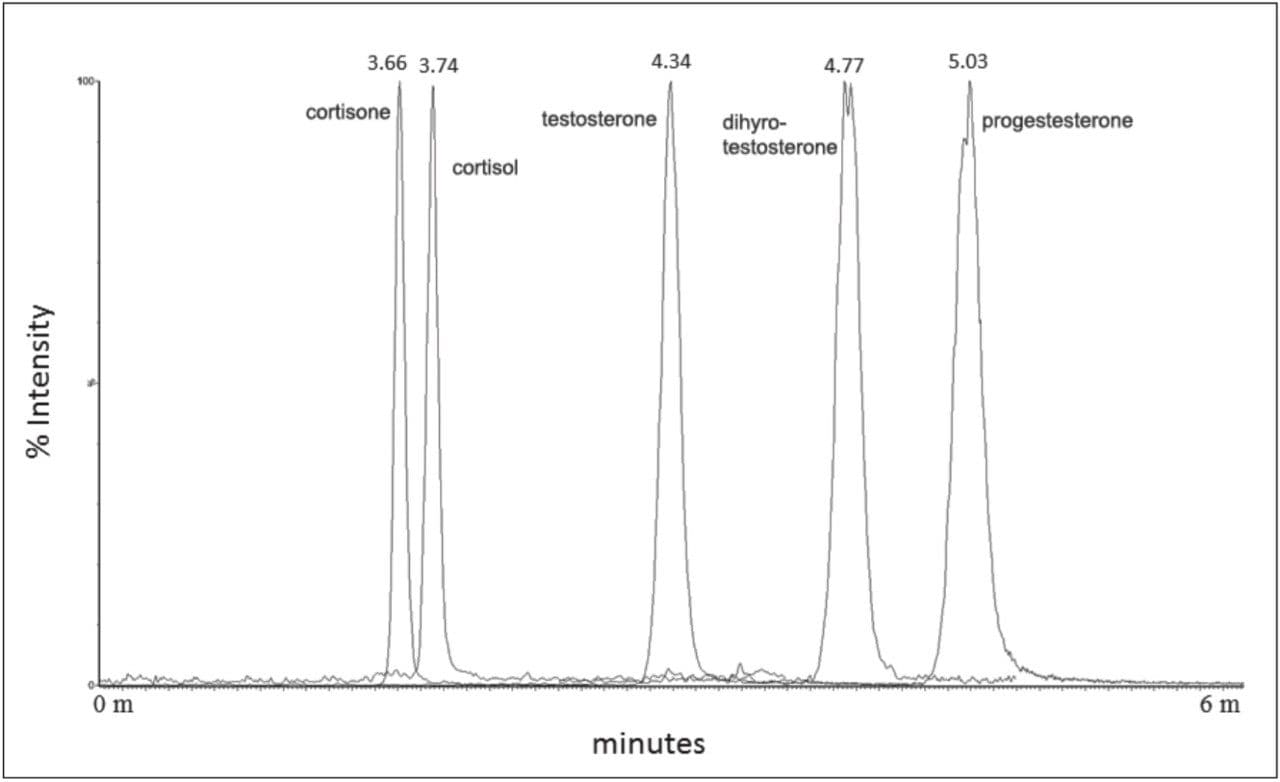
Chromatographic separation of the five compounds is illustrated in Figure 1. An average peak width of 6s was achieved.
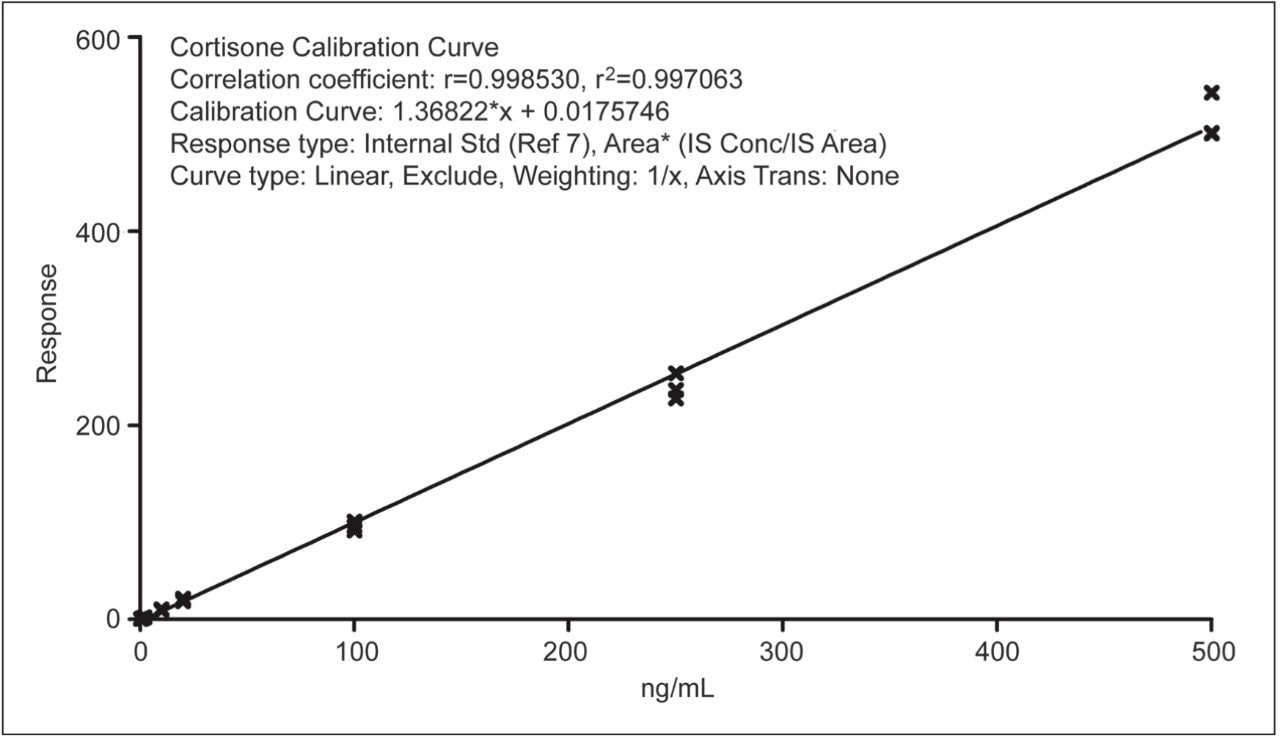
A typical calibration curve is shown in Figure 2. The correlation coefficients for all compounds were 0.99 or greater.
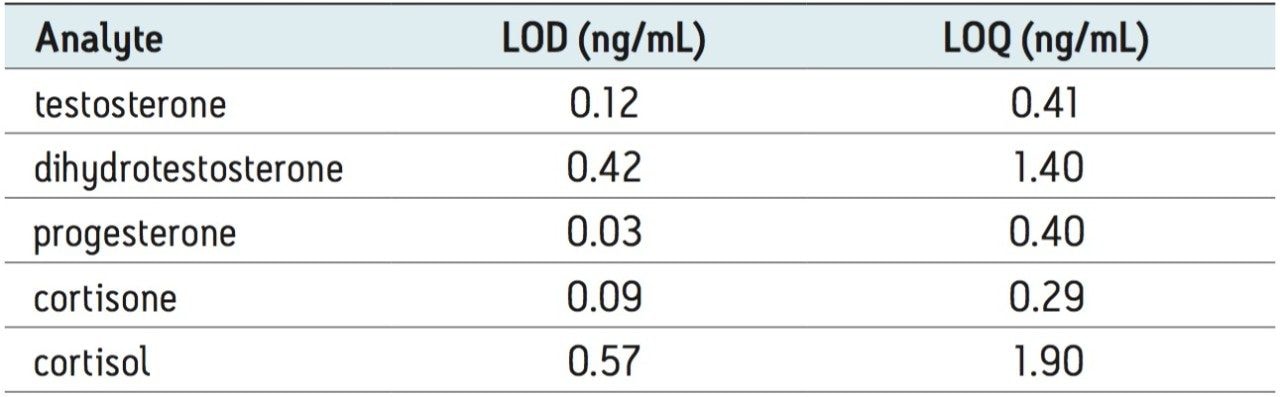
The lower limit of quantification (LOQ) and lower limit of detection (LOD) for all five compounds is listed in Table 2. These values represent a 100–400 fold increase in on-column analytical sensitivity as compared to published standard flow assays which typically require injection volumes of 50–200 µL of extracted sample;2,4 only 0.5 µL of extracted sample is used in the assay presented here.
720004956, January 2016