
This is an Application Brief and does not contain a detailed Experimental section.
Oral fluid is a highly valuable biological specimen and is commonly used in numerous settings, including therapeutic drug monitoring and testing. Oral fluid analysis has many advantages compared with other matrices such as blood or urine, such as collection convenience and reduced sample collection overheads.
The popularity of the specimen has led to the development of a large variety of collection devices, aiming to further simplify and standardize collection. Typically these devices will include additives and preservatives to improve the stability of the collected sample. One of the key analytical challenges with oral fluid analysis is the limited amount of sample available for testing compared with blood or urine, requiring very sensitive instrumentation to reach the low levels of detection and quantification. The ionKey/MS System offers the capability of improving sensitivity in sample-limited situations, making it ideally suited for this application.
In this technology brief, we demonstrate the separation and high sensitivity detection of opioids in oral fluid with the ionKey/MS System comprised of an ACQUITY UPLC M-Class System in combination with a Xevo TQ-S Mass Spectrometer.
Over the past three decades oral fluid has emerged as a highly valuable biological specimen and is commonly used in numerous settings, including therapeutic drug monitoring, and workplace and roadside drug testing. Oral fluid analysis has many advantages compared with other matrices such as blood or urine. These include collection convenience and reduced sample collection overheads.
Whilst oral fluid itself is a relatively clean matrix, comprising mostly of water and a small percentage of proteins, the popularity of the specimen has led to the development of a large variety of collection devices, aiming to further simplify and standardize collection. Typically these devices will include additives and preservatives to improve the stability of the collected sample. One of the key analytical challenges with oral fluid analysis is the limited amount of sample available for testing compared with blood or urine, requiring very sensitive instrumentation to reach the low levels of detection and quantification. The ionKey/MS System offers the capability of improving sensitivity in sample-limited situations, making it ideally suited for this application.

Performing analysis of oral fluid samples taken directly from a collection device provides a streamlined method for reducing workflow and increasing throughput. The use of a trapping column prior to analytical separation allows the removal of additives (e.g., surfactants) that can cause suppression in LC-MS and lead to reduced sensitivity. Trapping can also provide improved peak shape for hydrophilic small molecules, and crucially enables enhanced loading for ionKey/MS. Furthermore, the trapping column adds a layer of protection similar to that of a guard column, for precious downstream consumables, making the analytical method more robust.
Here we demonstrate the separation and high sensitivity detection of opioids in oral fluid with the ionKey/MS System comprised of an ACQUITY UPLC M-Class System in combination with a Xevo TQ-S Mass Spectrometer. Preliminary trapping was achieved using an ACQUITY UPLC M-Class HSS T3, 100Å, 1.8 μm, 300 μm x 50 mm Trap Column in combination with a 5 μL loop (Figure 1 and Table 1). Analytical separation was performed using an iKey HSS T3, 100Å, 1.8 µm, 150 µm x 100 mm Separation Device. Trapping-enabled quantitative analysis of 25 early eluting opioids and metabolites in oral fluid is illustrated in Figure 2.
Figure 3 shows the calibration curve and LOQ of morphine spiked into oral fluid. The LOQ (based on a signal to noise ratio of 10:1) was calculated to be 13 pg/mL. The peak width of morphine, at 10% peak height, was 3.0 seconds and is shown in the insert in Figure 2. Figure 4 compares morphine detected using an ionKey/MS System to an ACQUITY UPLC 2.1 mm I.D. Column. The ionKey/MS System shows an improvement of 9X over the analogous 2.1 mm column format. The trapping column and separation device used in these studies were specifically chosen for their increased retentivity for hydrophilic opioids and metabolites. These comparisons were performed with equivalent injection volumes of 5 μL.
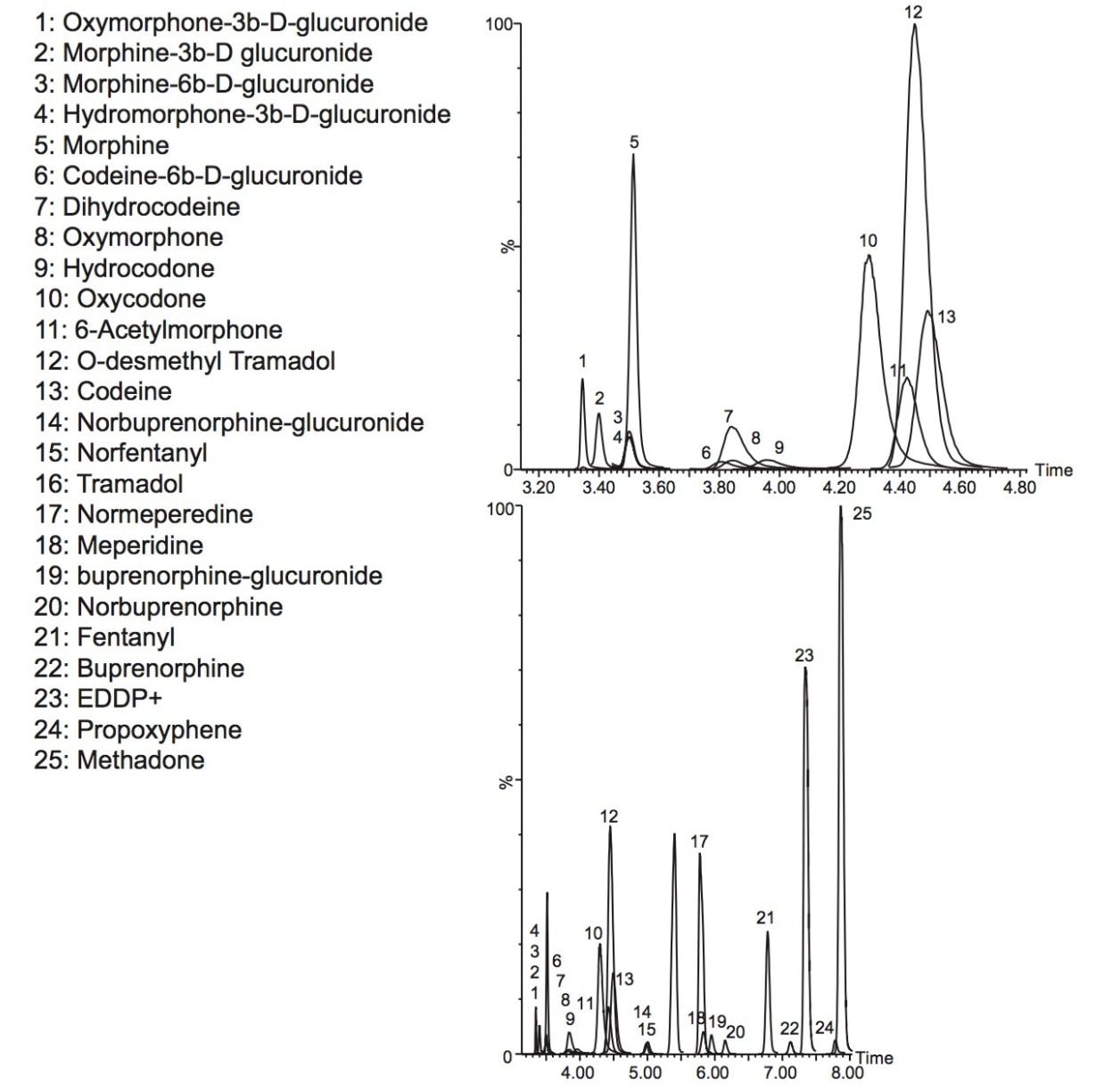
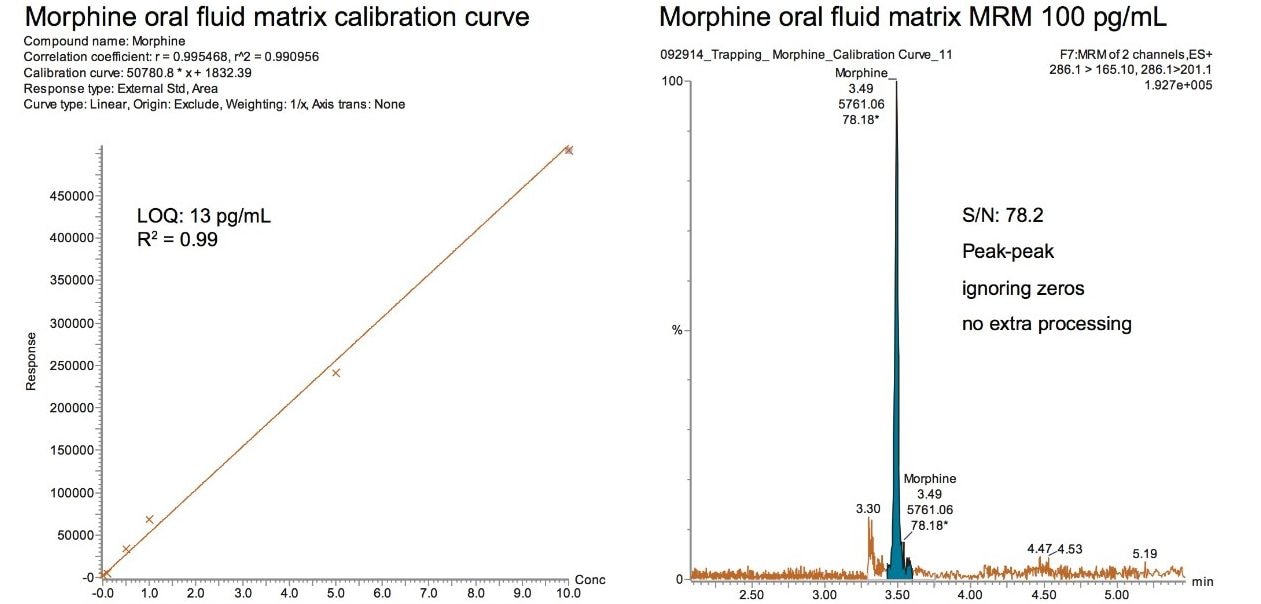
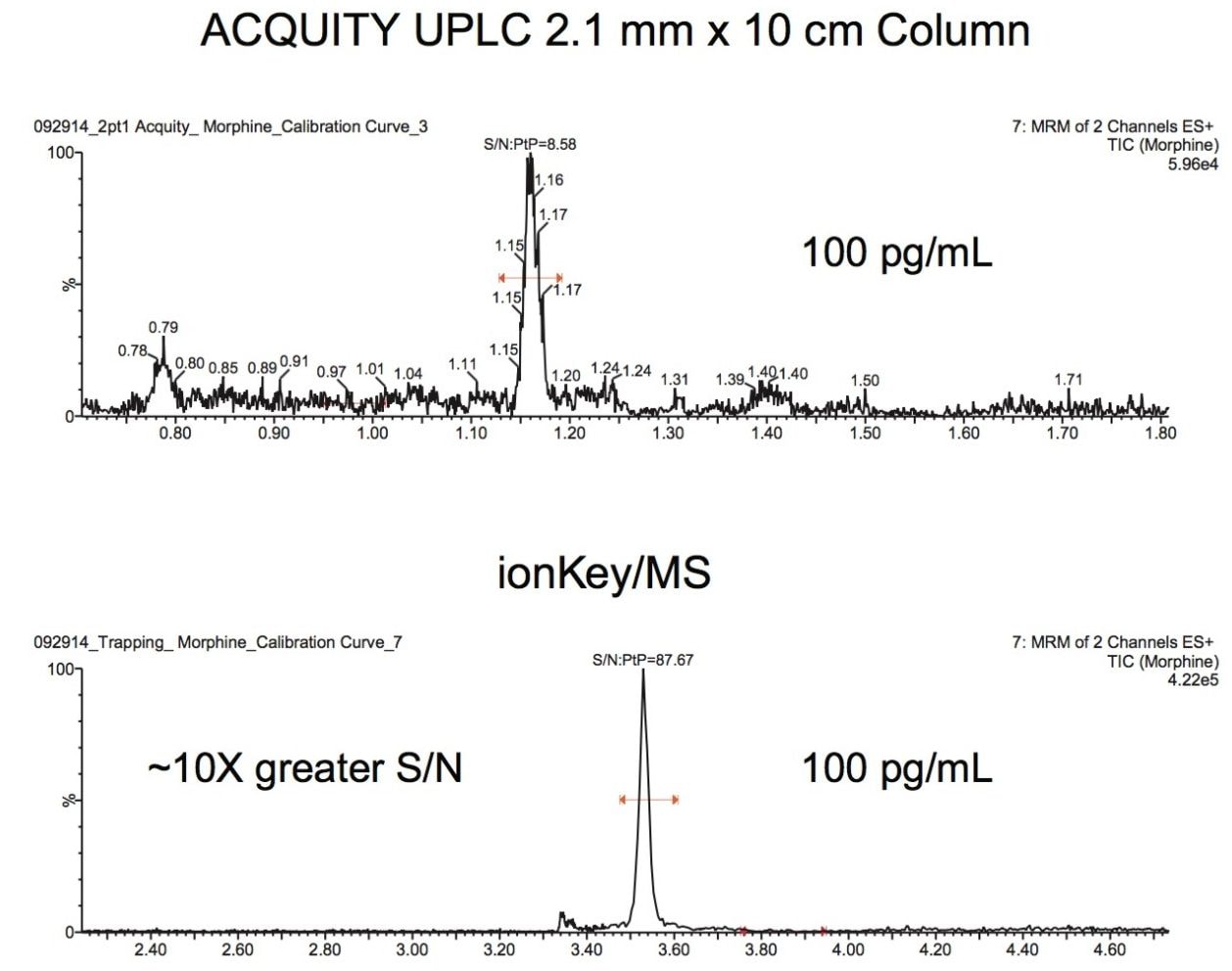
The utilization of the ionKey/MS System enabled a 9X improvement in sensitivity for morphine compared to an ACQUITY UPLC 2.1 mm I.D. Column when injecting the same volume. In addition to morphine, many of the glucuronides were also identified as having improved sensitivity.
Specifically, morphine-3b glucuronide was demonstrated to be baseline separated from morphine, with a sensitivity improvement of 11X. Whilst this particular glucuronide may not be relevant for oral fluid, the increased sensitivity presents a clear advantage for analysis in other biological specimens. The assay described here is robust and reproducible using oral fluid matrix injections of over 500 injections. Retention time reproducibilities were <1% RSD, while peak area reproducibility of morphine was 11.1% RSD.
720005296, July 2016