
This application note describes a more rapid chromatographic methodology for enantiomeric and diastereomeric separation and detection by using a combination of ACQUITY UPC2 and Trefoil chiral columns.
The development of analytical methods for the separation of chiral compounds is important in many areas of research, as it is well known that different enantiomers are selectively biologically active.1 Biochemical reactions can be diastereo or enantioselective. While one isomer may deliver the desired effect to the target species, the other enantiomer may be less effective to the target, completely ineffective, or cause undesirable effects. Additionally, it is known that different isomers can have very different environmental fates. It is estimated that 20 to 30% of pesticides on the market today have optical isomers, and there are reports that 40% of the pesticides used in China are chiral.1,2 The study of enantioselectivity is important to the crop protection industry, since the knowledge of the efficacy of each individual enantiomer could facilitate a significant reduction in the total amount of pesticide applied.
In order to improve our knowledge of the stereoisomeric compositions of these substances, analytical methods that provide reliable and reproducible separations in a rapid time frame are necessary. Supercritical fluid chromatography (SFC) is known as an effective chiral separations technique that has many advantages over conventional high performance liquid chromatography (HPLC).3,4 The properties of the supercritical fluid, such as low viscosity and high diffusivity, allow for the achievement of very high efficiency separations with shorter analysis times.5
In this application note we present the enantiomeric and/or diastereomeric resolutions of 12 triazole fungicides (Figure 1) using Waters Trefoil Column Technology. Trefoil Columns use a modified polysaccharide chiral stationary phase (CSP) with a 2.5 μm particle designed for broad-spectrum chiral selectivity. Resolutions were performed using an UltraPerformance Convergence Chromatography (UPC2) System. Convergence chromatography is a complimentary separation technique to liquid chromatography, that provides orthogonal selectivity, and uses supercritical CO2 as the primary mobile phase.
All separations were performed using the ACQUITY UPC2 System. Detection was by ACQUITY UPC2 Photodiode Array (PDA) Detector. Empower 3 Software was used for chromatographic data acquisition and processing.
Racemic pesticide standards were purchased from AccuStandard (New Haven, CT) The pesticide standards were prepared in methanol at a concentration of 1 mg/mL with the exception of cyproconazole and uniconazole which were purchased as 100 μg/mL stocks in methanol and acetonitrile respectively.
Method development for the stereoselective resolution of the technical grade fungicides began by using a generic screening gradient with a number of chiral columns and co-solvents, for example methanol, ethanol, 2-propanol, or mixtures of each. The ACQUITY UPC2 System has multi-column switching capabilities and a choice of four co-solvents. The screening step can be completed rapidly, due to the shorter analysis times that are possible using this technique. The combination of the co-solvent and column that produced the most promising separation for each compound was then selected for further optimization. The selectivity in a chiral separation can change markedly by varying the temperature, pressure, and flow rates.5
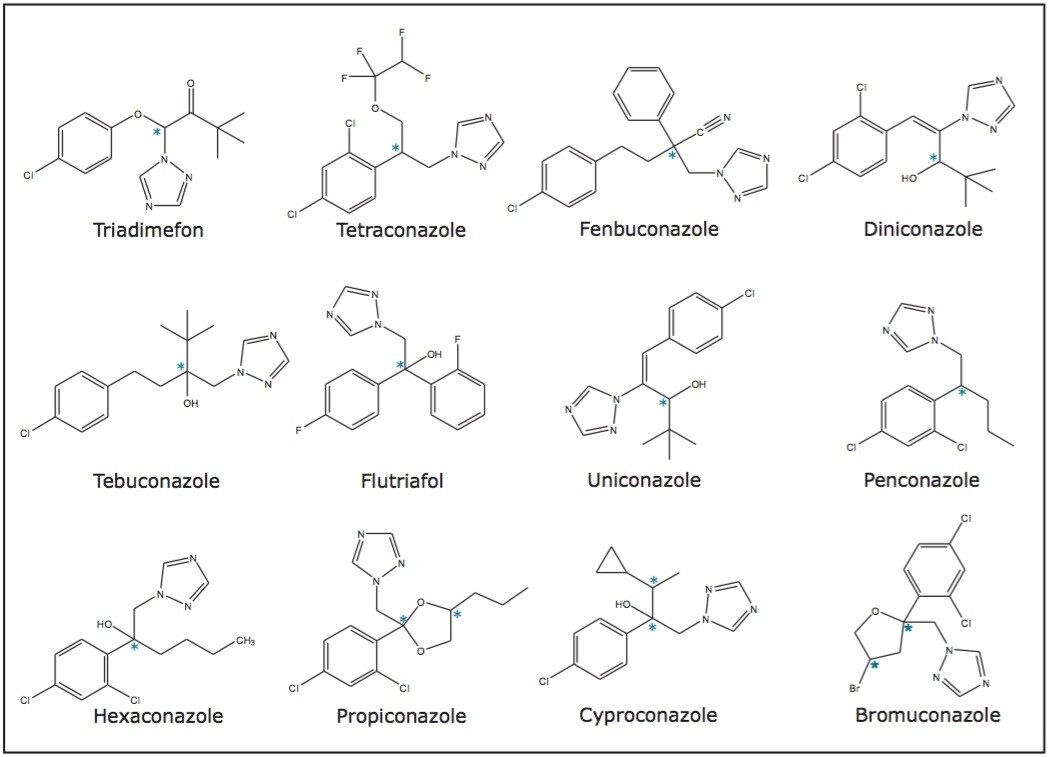
Separations in chiral chromatography typically result from multiple interactions between analytes and stationary phases. These interactions can be influenced differently by changing the experimental parameters to produce desired changes in the chromatography. Consequently, each parameter including temperature, pressure, and flow rate should be systematically evaluated to investigate the individual effects each change can have on the compound resolution. A summary of the selected analysis conditions is shown in Table 1.
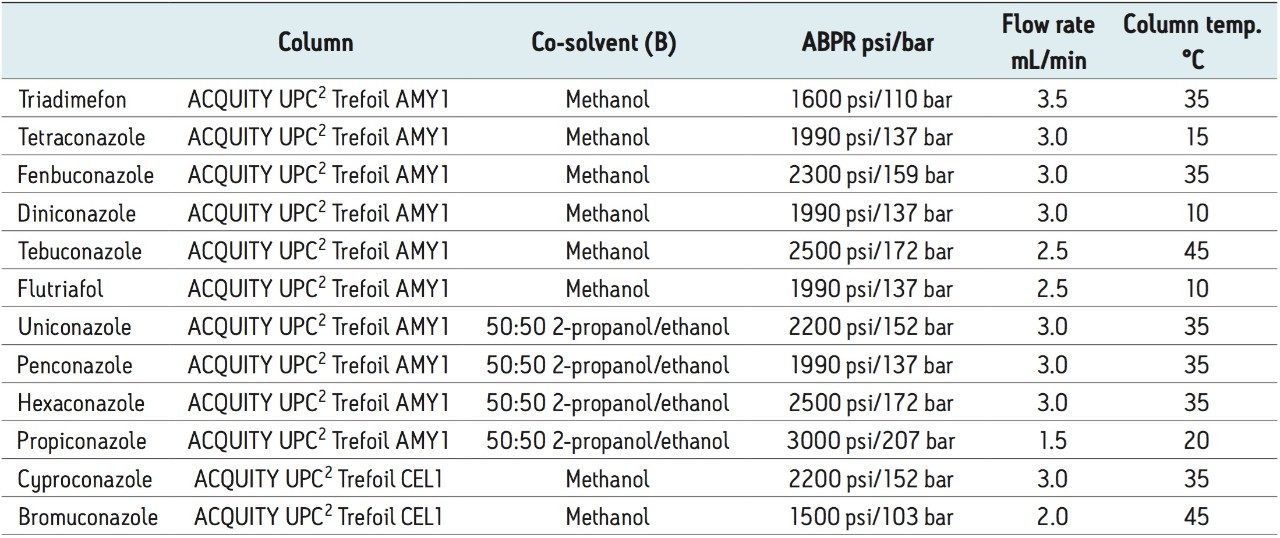
The chromatograms resulting from the optimized gradient separations of the racemic mixtures of triadimefon, tetraconazole, fenbuconazole, diniconazole, tebuconazole, and flutriafol are shown in Figure 2. In each case, the optimum column was a Trefoil AMY1 (3.0 x 150 mm, 2.5-μm , p/n 186007460), and the optimum co-solvent was methanol.
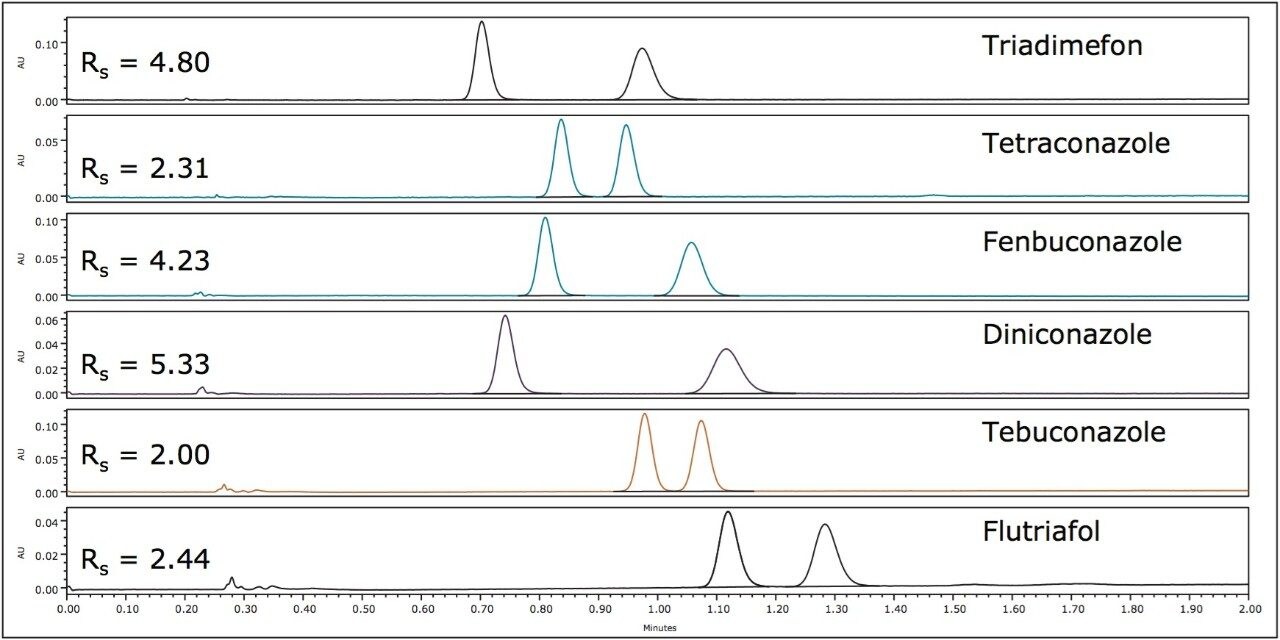
Baseline Rs was achieved for all pesticides in less than 1.5 minutes. Optimized resolutions for the racemic mixtures of uniconazole, penconazole and hexaconazole are shown in Figure 3. The optimum column in these cases was also a Trefoil AMY1, 3.0 x 150 mm, 2.5-μm, and the optimum co-solvent was 50:50 2-propanol/ethanol. Baseline resolution was achieved rapidly (less than 1.2 min) for the enantiomers of each triazole fungicide.
The chiral resolutions of propiconazole, cyproconazole, and bromuconazole, each with two chiral centers in their chemical structures, are shown in Figure 4. Despite the increase in the stereochemical complexity in the structures of these compounds, Rs values of >1.75 were achieved for all of the stereoisomers of each pesticide. The chiral separation of propiconazole is possible in less than 2.8 minutes using the ACQUITY UPC2 Trefoil AMY1 CSP Column (3.0 x 150 mm, 2.5-μm, p/n 186007464).
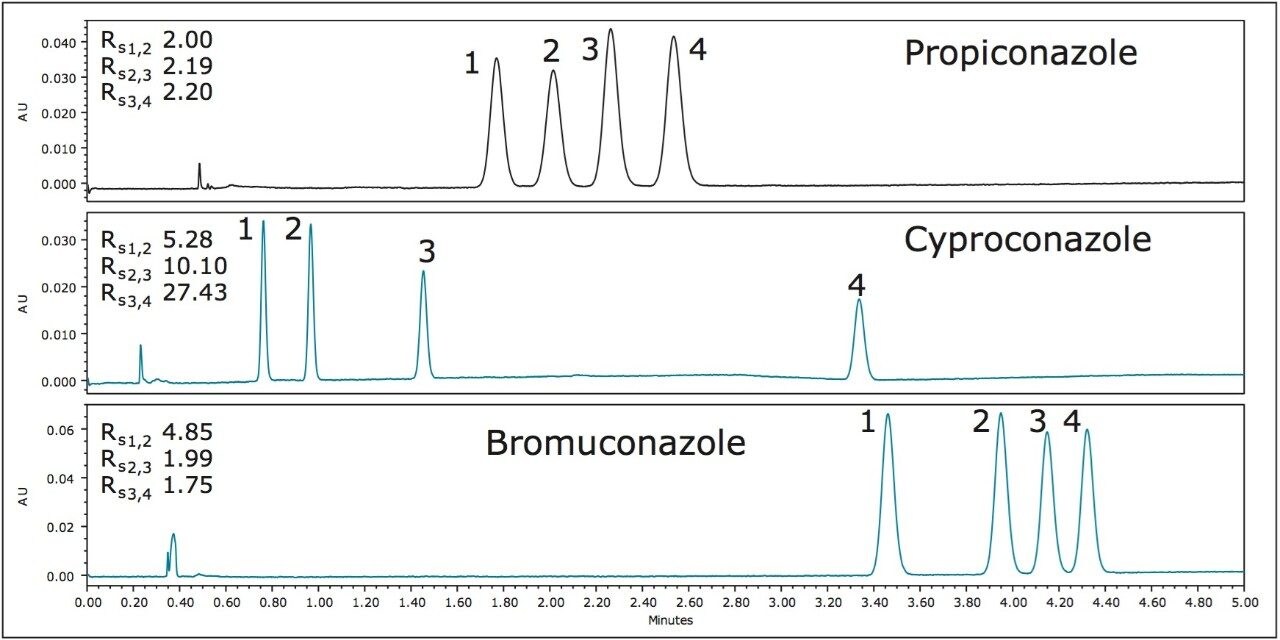
The optimum column in the case of cyproconazole and bromuconazole was the ACQUITY UPC2 Trefoil CEL1, 3.0 x 150 mm, 2.5-μm. The resolution of both triazole fungicides is possible in less than 4.5 minutes.
A review of the literature indicates that when using normal phase high performance liquid chromatography (HPLC), the chiral resolution of propiconazole is possible in 34 min, and the enantiomeric resolution of tebuconazole ranged from 17 to 45 min.6-11 Similar resolutions were achieved for propiconazole and tebuconazole using traditional SFC, but the analysis times were reduced to 10 minutes and 10.5 minutes, respectively.4
The literature search also revealed two reviews6,8 showing that the chiral resolutions of the test compounds using UPC2 can be achieved much faster compared to reverse phase12-19 (3 to 30X), normal phase6-11 (8 to 40X), and conventional SFC4,20 (3 to 10X) separations.
The optimized ACQUITY UPC2 methods developed in this work allow increased sample throughput and improved enantiomeric resolutions, especially when compounds with multiple chiral centers are analyzed.
Reproducibility data (n=8) for retention time, area, area%, height, and USP resolution for bromuconazole are shown in Table 2. The %RSD’s were less than or equal to 0.60% for all of the stereoisomers.
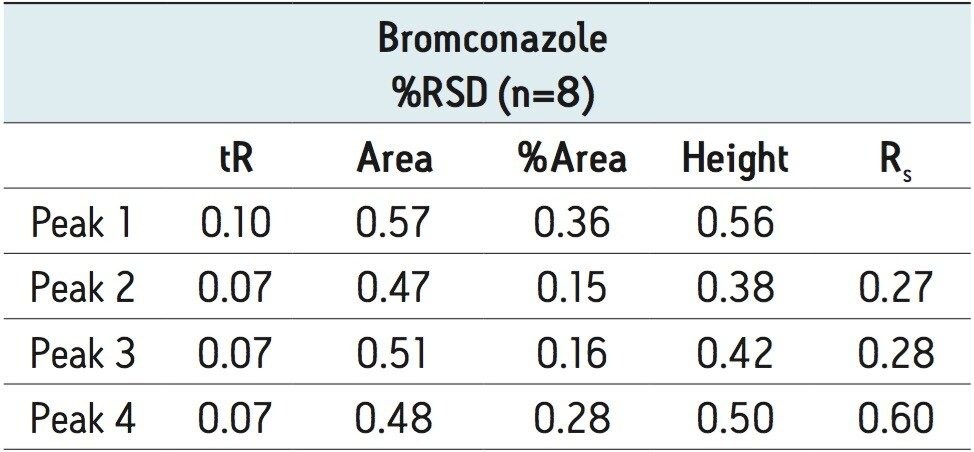
The study of enantioselectivity is important to the crop protection industry since the knowledge of the efficacy of a more biologically active individualenantiomer could facilitate a significant reduction in the total amount of pesticide applied and result in a more marketable product. The rapid enantioseparation of chiral pesticides has previously been challenging due to the difficulty in chromatographically resolving them in short analysis times. This application note highlights a more rapid chromatographic methodology for enantiomeric and diastereomeric separation and detection by using a combination of ACQUITY UPC2 and Trefoil chiral columns. The result was a highly efficient stereoselective separation of 12 triazole fungicides using two CSP’s. Further, the methodology shown in this work improves the sample throughput compared with LC-based chiral separations.5-19 The %RSD’s (n=8) for retention time, area, area%, height, and USP resolution for bromuconazole were less than or equal to 0.60% for all of the stereoisomers. These methods use supercritical CO2 as the primary mobile phase and alcohol modifiers as the co-solvents. The need to use large volumes of potentially hazardous solvents that are routinely used in normal phase chiral separations is reduced, as well as the cost associated with solvent waste disposal.
720005404, May 2015