In this application note, we have used the ProteinWorks eXpress Direct Digest Kit to simplify and streamline the workflow process using the same universal protocol and reagents for all monoclonal antibody drugs tested.
The ProteinWorks eXpress Direct Digest Kit was successfully used to purify and simultaneously quantify infliximab, adalimumab, bevacizumab, and trastuzumab from a typical set of standard curve and QC samples in rat plasma. The standardized, kit-based approach enables inexperienced users to immediately obtain meaningful data in discovery studies in order to make time sensitive and critical project decisions.
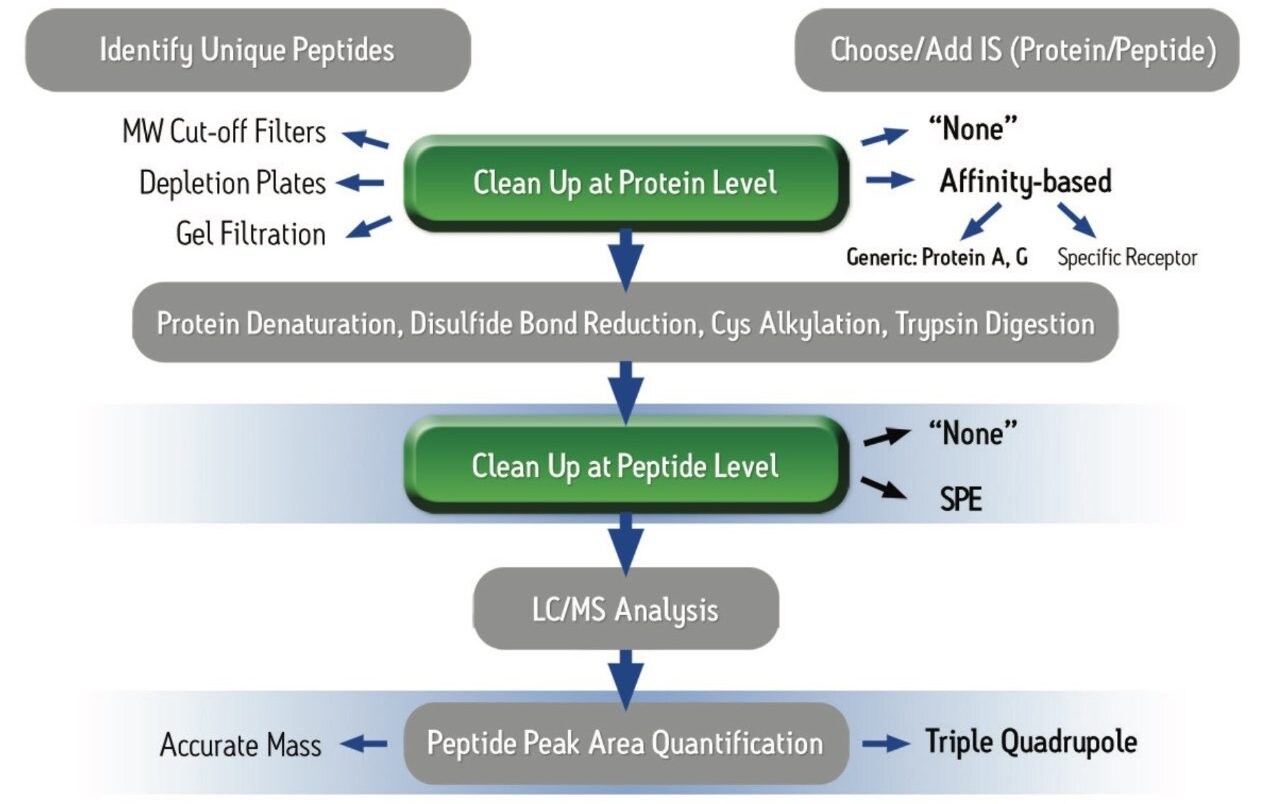
Over the past 5–10 years, there has been a significant shift towards a greater % of biologics in pharmaceutical pipelines.1 However, the industry finds itself in the middle of patent expiry for many of the critical monoclonal antibody and other protein-based drugs, with patent expiration dates ranging from ~2012–2020.2 This has resulted in a focus on protein quantification in bioanalytical labs, innovator pharma and CRO’s as well as biomarker research labs. While immunoassay (IA) methods are sensitive and simple to execute, poor reagent reproducibility, lack of standardization, cross-reactivity, limited linear dynamic range, and other short-comings have led the drive to convert to LC-MS. These LC-MS workflows however, encompass a multitude of sub-segments, each having many steps. Those that are common to most workflows may include affinity purification, denaturation, reduction, alkylation, digestion, and SPE clean-up (each requiring optimization). Such traditional protein quantification protocols often require as much as a day and half for completion. Furthermore, the margin and possibility of error is significant within each individual step. There is a strong need for simpler, more standardized workflows which enable scientists to complete sample preparation and start an analytical run by mid-day. At the same time, ideally using generic, kitted methods, assay sensitivity must be high enough to accurately and precisely quantify low enough levels of the target protein to make critical decisions in discovery. The typical workflow complexity as shown in Figure 1, often leads to errors and poor reproducibility or sensitivity. In this application note, we have used the ProteinWorks eXpress Direct Digest Kit to simplify and streamline the workflow process using the same universal protocol and reagents for all monoclonal antibody drugs tested. Infliximab, bevacizumab, trastuzumab, and adalimumab (Figures 2–5) in plasma were directly digested, and peptides extracted using SPE in under 4 hours total time. This enabled data to begin to be acquired the same day, with several 96-well plates being run by the following morning.
Infliximab, adalimumab, bevacizumab, or trastuzumab were spiked into human plasma. 35 μL plasma samples were then prepared for LC-MS analysis using the ProteinWorks eXpress Direct Digest Kit and Protocol. After digestion, signature peptides were cleaned-up using the ProteinWorks μElution SPE Clean-up Kit and Protocol.
|
LC System: |
ACQUITY UPLC |
|
Detection: |
Xevo TQ-S Mass Spectrometer, ESI+ |
|
Column: |
ACQUITY UPLC Peptide BEH C18, 300 Å, 1.7 μm, 2.1 mm x 150 mm |
|
Column temp.: |
55 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Capillary (kv): |
3 |
|
Cone (V): |
30 |
|
Source offset (V): |
50 |
|
Source temp. (°C): |
150 |
|
Desolvation temp. (°C): |
600 |
|
Cone gas flow (L/hr): |
150 |
|
Desolvation gas flow (L/hr): |
1000 |
|
Collision gas flow (mL/min): |
0.15 |
|
Nebulizer gas flow (Bar): |
7 |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
0 |
0.3 |
100 |
0 |
6 |
|
1 |
0.3 |
100 |
0 |
6 |
|
7 |
0.3 |
50 |
50 |
6 |
|
8 |
0.3 |
10 |
90 |
6 |
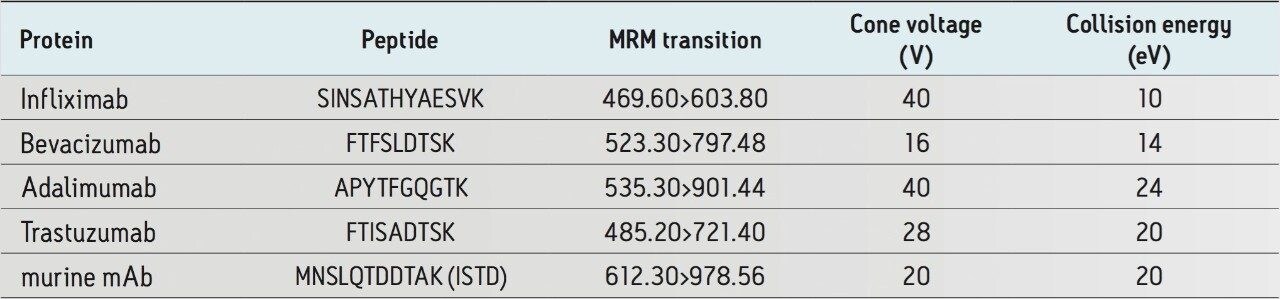
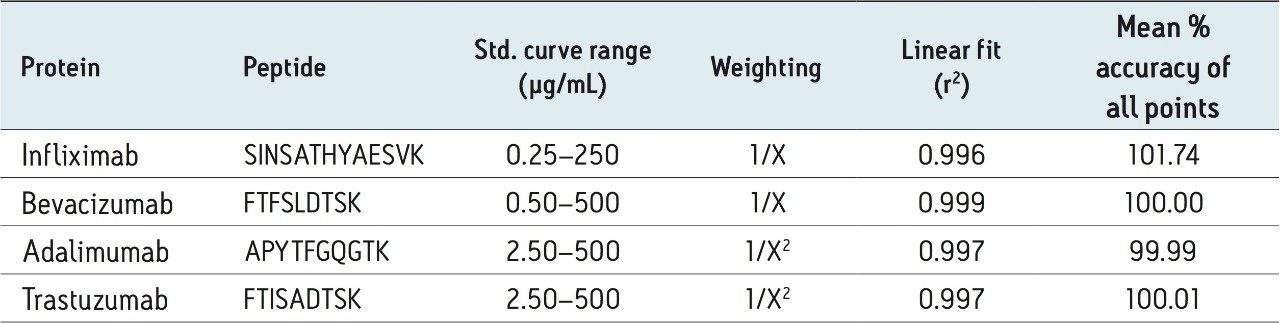
In a pre-clinical setting, there is an emphasis on simple, broadly applicable, generic protocols as method development time and expertise are at a premium. Multiple signature peptides were used to quantify 4 different monoclonal antibody drugs in human plasma using direct digestion and the ProteinWorks eXpress Direct Digest Kit. For each protein, sensitivity, linearity, accuracy and precision data all met typical method validation requirements using the same broadly applicable ProteinWorks Kit. Through a direct digest of a 35 µL plasma sample, quantification limits ranged from 250 ng/mL–2.5 µg/mL for the 4 monoclonal antibody-based drugs. Standard curves were linear over 3.5–4 orders of magnitude with the average accuracies for standard curve points typically within 95–105%. Summary statistics from standard curves for infliximab, adalimumab, trastuzumab, and bevacizumab are shown in Table 2 below.




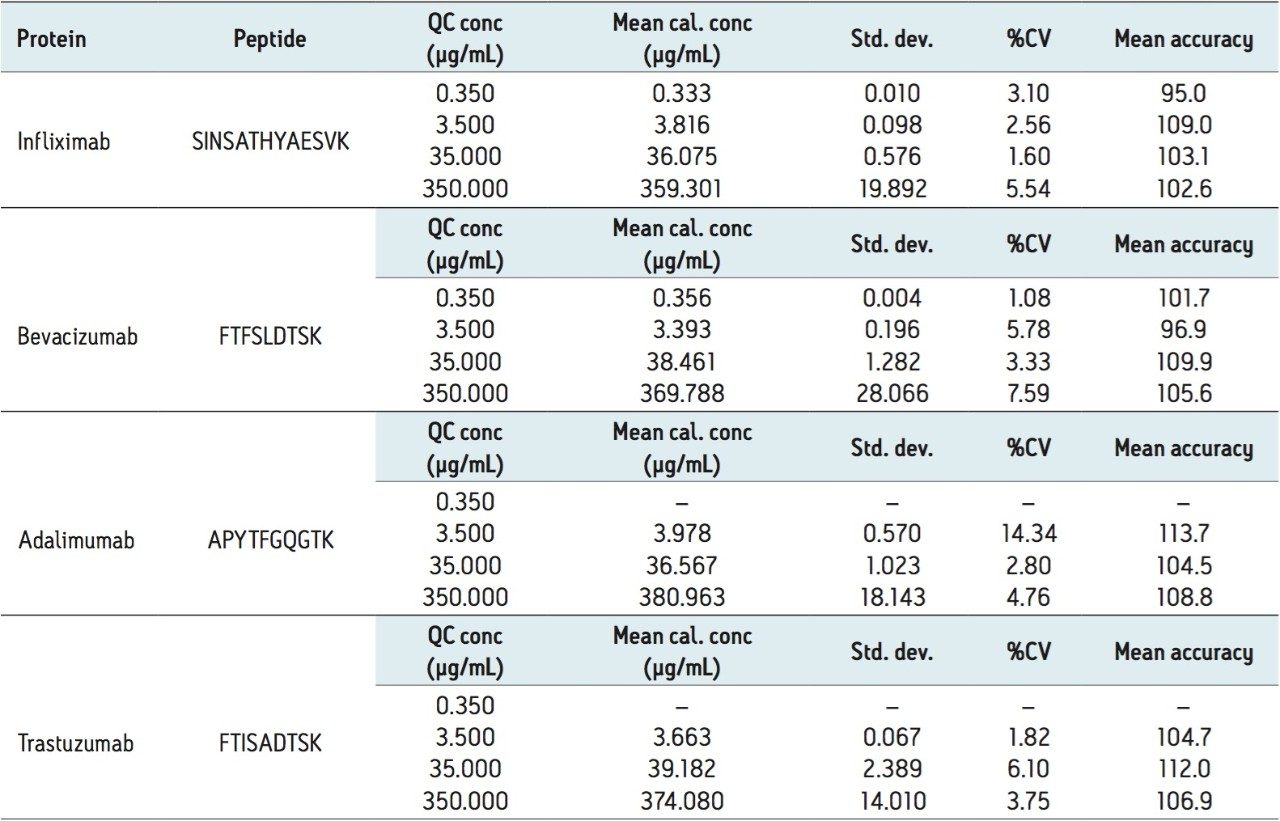
At the same time, QC statistics (summarized in Table 3 below) also easily met regulatory guidelines,3 with average precision values well under 15%, and averaging in the single digits.
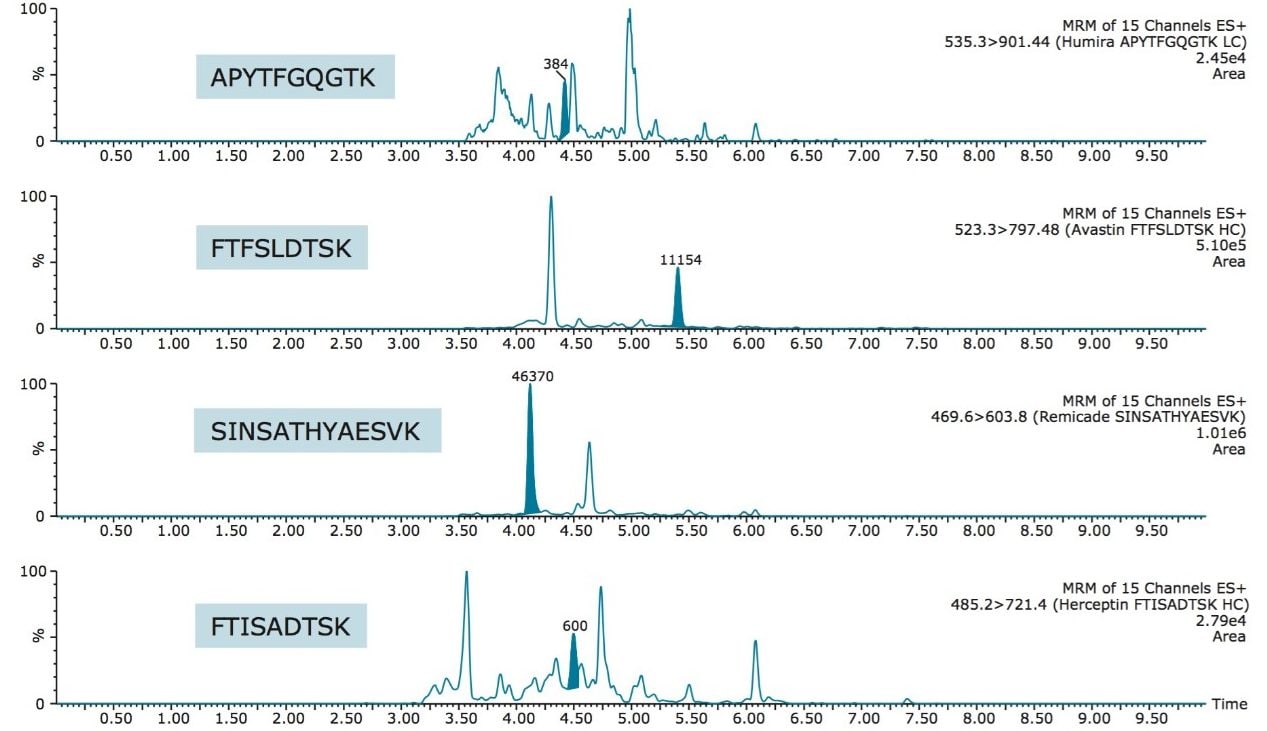
For a typical discovery study, detection limits ~1 µg/mL are common for monoclonal antibody type drugs. Using the ProteinWorks eXpress Direct Digest kit, these limits are easily obtained for all 4 drugs evaluated. Low QC chromatograms are shown in Figure 6 which demonstrate that adequate sensitivity is achieved with a single universal protocol and the kit.
In this study, the single universal digest protocol and SPE method designed for tryptic peptides eliminated the need for discovery-stage method development. The fact that the kit was able to accurately and precisely quantify 4 monoclonal antibody drugs in plasma without the need for optimization demonstrates its broad applicability and utility in an environment where time is critical and experience with protein bioanalysis may be limited. Furthermore, the application of a kit with lot-traceable, pre-measured reagents ensures that methods may be seamlessly transferred across sites and labs, or analysts.
The ProteinWorks eXpress Direct Digest Kit was successfully used to purify and simultaneously quantify infliximab, adalimumab, bevacizumab, and trastuzumab from a typical set of standard curve and QC samples in rat plasma. Quantification limits of 250 ng/mL to 2.5 µg/mL for each antibody were readily achieved, while maintaining excellent linearity and precision. The total sample prep time including digestion and SPE was just over 3 hours. The standardized, kit-based approach enables inexperienced users to immediately obtain meaningful data in discovery studies in order to make time sensitive and critical project decisions.
720005541, November 2015