The first study focuses on the achiral analysis and purification of a reaction intermediate and product for the synthesis of the active pharmaceutical ingredient (API), Imatinib. The second study is for the purification of a proprietary pharmaceutical chiral compound. The ability to scale methods predictably and efficiently enables the rapid screening of methods on the faster analytical scale, with the direct transfer of the final method to preparative chromatography while maintaining chromatographic integrity between separations. The net result is a significant savings in time and mobile phase costs (raw materials and disposal of waste).
In a previous application note, the use of density modulation was presented as an effective strategy for scaling SFC separations for preparative SFC purification.1 This strategy is rooted in the principle that, for separations utilizing CO2 as the principal mobile phase component, analyte retention factors are influenced largely by the mobile phase density and temperature. Modulation of the mobile phase density for the scaled separation, to approximate the average density of the original separation, yields a predictable separation that maintains the chromatographic integrity of the original separation. Modulation of the density can be accomplished by adjusting, in an appropriate way, any of the parameters which would impact the overall system pressure of the separation, and therefore the density of the mobile phase; e.g., the automated back-pressure regulator (ABPR) setting, the flow rate, or the column configuration. Of these options, adjusting the ABPR is more convenient and will be used as an example in this application note. In some cases, scaling by maintaining the same ratio of column length to particles size (L/dp), which is a common practice for LC scaling methodologies, is a useful first step for modulating density for scaling of SFC methods. In each case, density simulations can be used to understand the density profiles for the separations so that density modulation can be accomplished in a systematic manner. In this application note, we execute this strategy in real-life applications, highlighting two case studies, using the ACQUITY UltraPerformance Convergence Chromatography (UPC2) System for the fast development of methods on the analytical scale with the subsequent scale-up to preparative SFC purification. The first study focuses on the achiral analysis and purification of a reaction intermediate and product for the synthesis of the active pharmaceutical ingredient (API), Imatinib. The second study is for the purification of a proprietary pharmaceutical chiral compound. The ability to scale methods predictably and efficiently enables the rapid screening of methods on the faster analytical scale, with the direct transfer of the final method to preparative chromatography while maintaining chromatographic integrity between separations. The net result is a significant savings in time and mobile phase costs (raw materials and disposal of waste).
Sample preparation
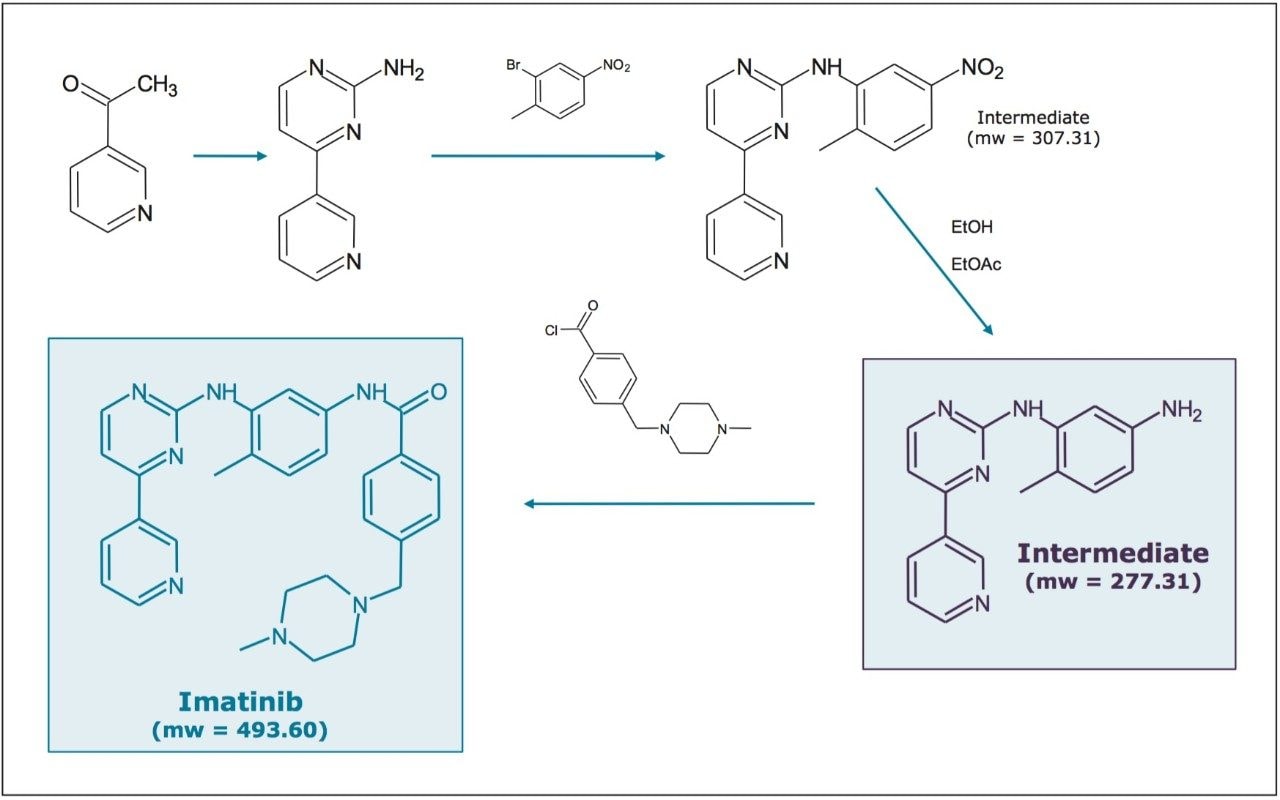
The final intermediate and product were collected from the reactions, shown in Figure 1, as part of the chemical synthesis of Imatinib, a tyrosine-kinase inhibitor used in the treatment of multiple cancers.2,3 Samples for preparative purification were prepared at a concentrations of 10 mg/mL with a diluent of 2-propanol/hexane/DMSO/acetonitrile (40:13:27:20). An additional dilution to 0.2 mg/mL with the same diluent was used for analytical injections.
|
UPC2 conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Columns: |
ACQUITY UPC2 BEH 2-Ethylpyridine, 1.7 μm, 2.1 x 50 mm, p/n: 186006576 ACQUITY UPC2 BEH, 1.7 μm, 2.1 x 50 mm, p/n: 186006558 ACQUITY UPC2 CSH Fluoro-Phenyl, 1.7 μm, 2.1 x 50 mm, p/n: 186006567 |
|
Mobile phase A: |
CO2 (tank, medical grade) |
|
Mobile phase B: |
Methanol or methanol with 0.3% ammonium hydroxide (NH4OH) |
|
Flow rate: |
1.5 mL/min |
|
Gradient: |
4–40% Modifier in 2 minutes (~26 column volumes) |
|
Column temp.: |
40 °C |
|
ABPR: |
1800 psi |
|
UV detection: |
235 nm (Compensated 380–480 nm) [40 pts/sec] |
|
Injection volume: |
2.0 μL |
|
Strong needle wash: |
2-Propanol (IPA) |
|
Weak needle wash: |
2-Propanol (IPA) |
|
Seal wash: |
2-Propanol (IPA) |
|
Vials: |
LCMS Certified Max Recovery Vials |
|
System: |
Prep 100q SFC System with PDA and MS detection |
|
Columns: |
Viridis BEH 2-Ethylpyridine OBD Prep, 5 μm, 19 x 150 mm, p/n: 186005764 |
|
Mobile phase A: |
CO2 (house CO2 delivery system) |
|
Mobile phase B: |
Methanol with 0.3% ammonium hydroxide (NH4OH) as additive |
|
Flow rate: |
80 mL/min |
|
Gradient: |
4–40% Modifier in 9.2 minutes (~26 column volumes) |
|
Column temp.: |
40 °C |
|
ABPR: |
1800 psi |
|
UV detection: |
235 nm |
|
Injection volume: |
120 μL |
|
Wash solvent: |
Methanol |
Sample preparation
The sample is a proprietary chiral compound that degrades readily on exposure to water or alcohols. For that reason, the sample was prepared using acetonitrile as the diluent. The ability to address water-labile compounds is one of the additional benefits of SFC.
|
UPC2 conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 with ACQUITY UPC2 PDA Detector |
|
Column: |
Cellulose tris 3,5- dimethylphenylcarbamate stationary phase, 3 μm, 4.6 x 150 mm |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Acetonitrile |
|
Flow rate: |
2 mL/min |
|
Gradient: |
20–50% Modifier in 3 minutes (~3.6 column volumes) |
|
Column temp.: |
40 °C |
|
ABPR: |
2175 psi |
|
UV detection: |
212 nm |
|
Injection volume: |
1.0 μL |
|
Strong needle wash: |
2-Propanol (IPA) |
|
Weak needle wash: |
2-Propanol (IPA) |
|
Seal wash: |
2-Propanol (IPA) |
|
System: |
Prep 100q SFC system with PDA and MS detection |
|
Column: |
Cellulose tris 3,5- dimethylphenylcarbamate stationary phase, 5 μm, 21 x 250 mm |
|
Mobile phase A: |
CO2 (house CO2 delivery system) |
|
Mobile phase B: |
Acetonitrile |
|
Flow rate: |
70 mL/min |
|
Gradient: |
20–50% Modifier in 2.8 minutes (~3.6 column volumes) |
|
Column temp.: |
40 °C |
|
ABPR: |
1827 psi |
|
UV detection: |
212 nm |
|
Injection volume: |
300 μL |
The simulation of the density profiles presented here was conducted based on the assumption that the variation of the pressure profile along the column is linear. The densities of the CO2/methanol mixtures were calculated using the REFPROP software from NIST.4 REFPROP calculates the neat CO2 density following the Span and Wagner equation of state (EOS) and calculates the CO2/MeOH mixture density using the Kunz and Wagner model.5,6 For gradient methods, the density not only varies along the column, but also changes with time. The increasing modifier concentration during the gradient results in increases in mobile phase viscosity and therefore pressure, which in turn impacts the mobile phase density profile. For gradient separations, the density modulation strategy should consider the changing density profile as the modifier transitions from lower to higher concentrations.
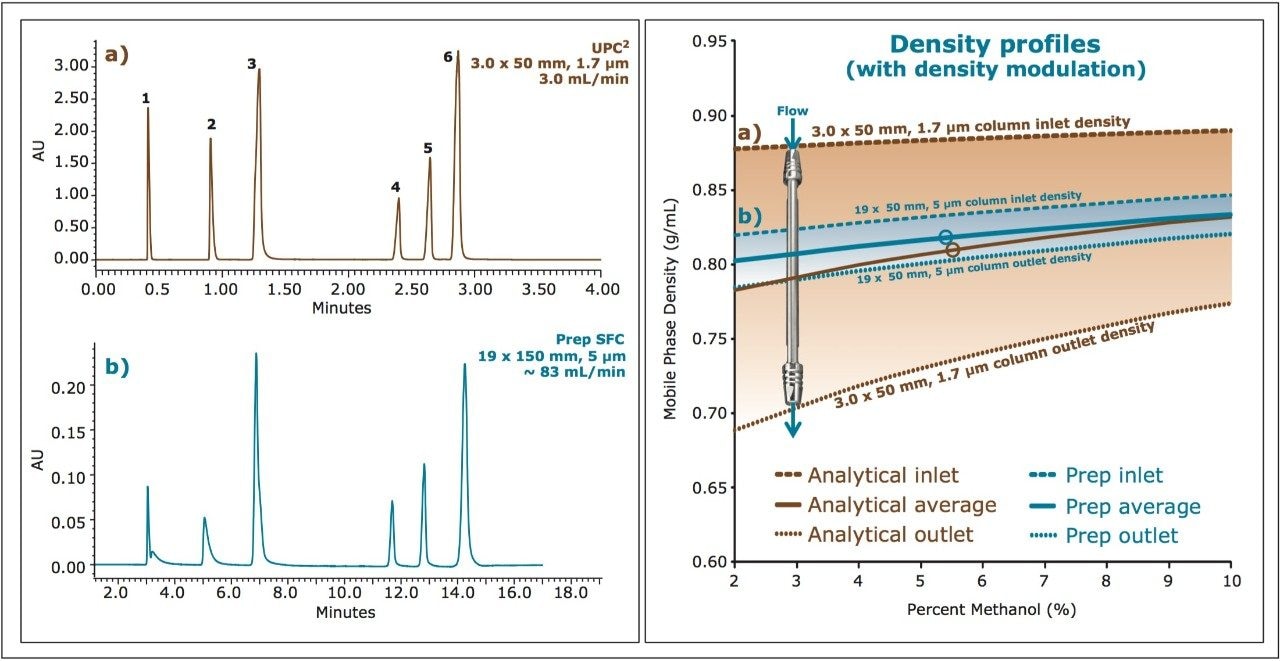
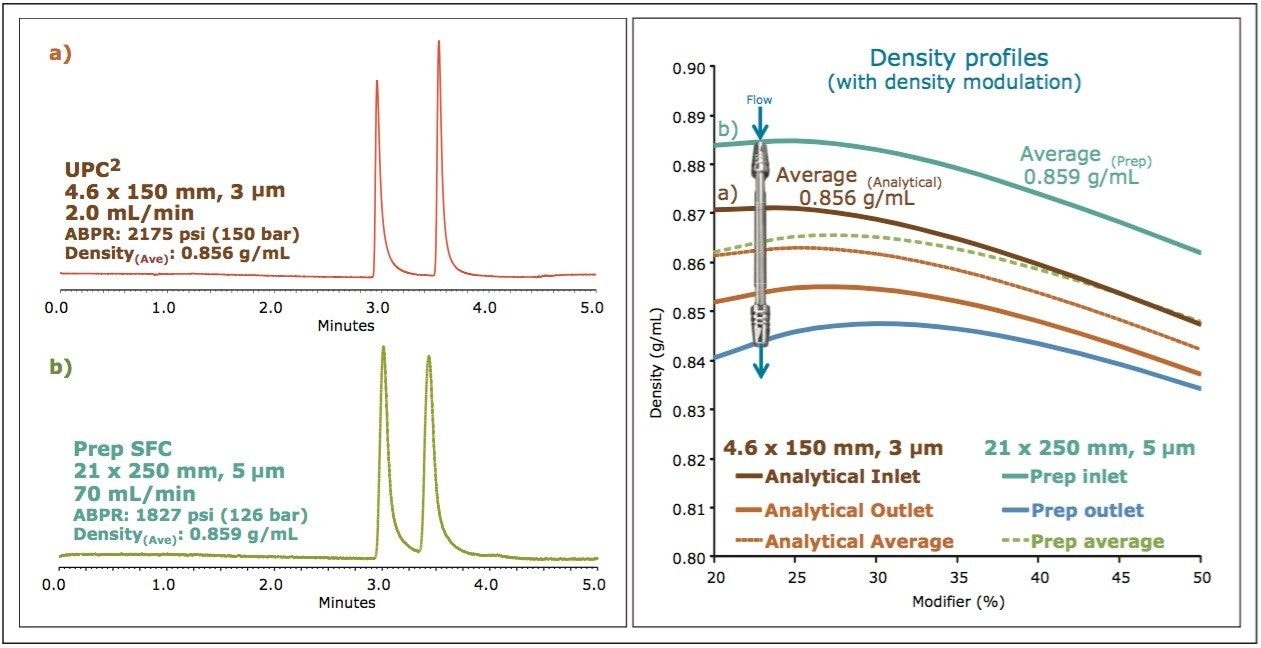
Figure 2 shows an example of this approach for a mixture of standards separated at the analytical scale and at the preparative scale with modulated density. The density profiles were calculated for the inlet and outlet of the columns, for both the analytical and preparative configurations. For the preparative configuration, the density was modulated to yield approximately the same average density profile as that calculated for the analytical configuration. The resulting chromatography (Figure 2, left) demonstrates the ability to achieve similar separations with this approach, yielding similar resolution and selectivity for the individual compounds. Differences observed between the two examples can be attributed to differences in system dwell volume or injection mode (modifier stream vs. mixed stream injection), neither of which were considered for these experiments.
For the initial method development, samples of the intermediate and product were screened on three different ACQUITY UPC2 Column chemistries using methanol as a modifier, with a generic screening gradient of 4-40% modifier. Not surprisingly, due to the amine functionalities on both compounds, severe tailing was observed for the intermediate, while no elution of the product was observed on two of the UPC2 Column chemistries. Significant improvement in peak shape was observed when compounds were re-screened using an additional basic additive of 0.3% ammonium hydroxide in the methanol modifier. The screening results are shown in Figure 3.
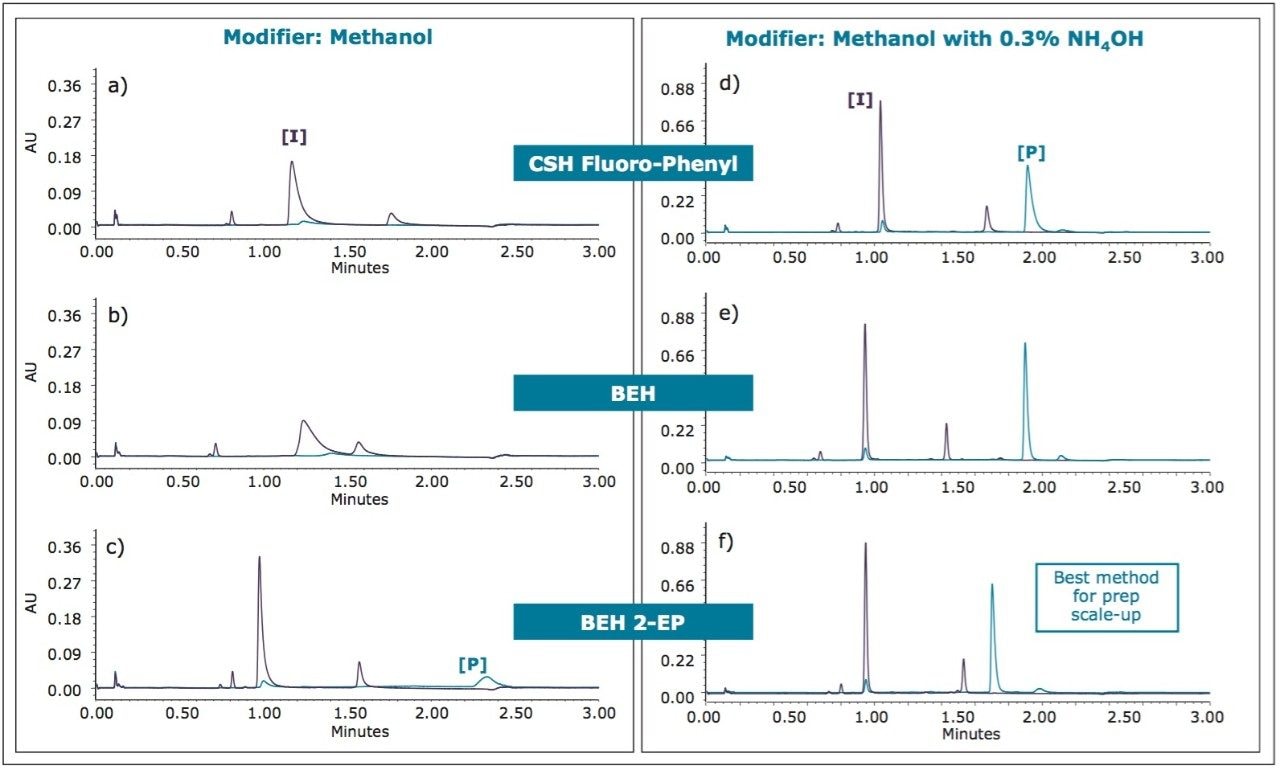
Based on these initial results, the BEH 2-Ethylpyridine chemistry was chosen for additional evaluations in scaling up for preparative purification, using a modifier of methanol with 0.3% ammonium hydroxide as additive.
As previously discussed, to scale SFC separations in a predictable manner, it is critical to understand the density profiles for the individual separations. For the selected screening gradient shown in Figure 3(f), the density was simulated for both the column inlet and outlet at multiple points during the gradient, as the modifier transitioned from 4% to 40%. From these values, the average mobile phase density that would be experienced by an analyte during the separation was calculated to be 0.857 g/mL. For scaling to the preparative scale, the strategy of maintaining the same ratio of column length to particle size (L/dp) was employed. Based on the 1.7 μm, 50 mm analytical column dimension, a preparative column was chosen in a 5 μm, 19 x 150 mm configuration. The method was scaled geometrically for separation on a Prep 100q SFC System equipped with both UV and MS detection. A preparative flow rate of 80 mL/min was chosen based on the optimum performance of the system at that flow rate. The gradient time (tg) was scaled from the analytical conditions such that the total number of column volumes (CV) for each gradient were the same (~26 CV), resulting in a preparative gradient of 9.2 minutes. The gradient modifier concentrations were the same as for the analytical separation (4-40%). Chromatograms for both the intermediate [I] and product [P] were collected, at concentrations of 10 mg/mL, with the preparative method. As was performed for the analytical separation, the density profiles were calculated for the preparative column inlet and outlet at multiple points during the separation, taking into account the changing density of the mobile phase with changing concentration of the modifier. From those simulations, the average density for the preparative separation was calculated to be 0.847 mg/mL. The density simulations for the analytical and preparative scale, along with the resulting chromatography are shown below in Figure 4.
Based on the geometric scaling used for the method, with the column configurations chosen to maintain L/dp, the resulting average densities calculated for the two separations were very similar, resulting in similar chromatography without the need for additional density modulation (see Figure 4a and 4b). While this is true for this particular example, maintaining L/dp may not always yield similar average densities, due to differences in flow rates. Only through the density simulations can this be confirmed.
![Evaluation of the intermediate [I] and product [P] from the synthesis of Imatanib.](/content/dam/waters/en/app-notes/2014/720005064/720005064en-f4.jpg.82.resize/img.jpg)
The ideal separation scenario for purification is the fast screening of a method at the analytical scale to establish purification conditions, with a subsequent direct scale-up to a preparative purification using a focused gradient to target a specific analyte of interest. Focused or segmented gradients are commonly used in LC separations. However, for SFC separations, where mobile phase density plays a critical role, their use is not as well understood. To understand this better, the analytical screening separation (shown in Figure 3f) was used to develop an analytical scale focused gradient separation. This intermediate step of developing an analytical focused gradient method was done solely to test the concept of a focused gradient. In our ideal scenario, this intermediate step would not be required. While the resulting methods would be the same, it is important to note that the conditions to be used for the focused gradient at the preparative scale are derived from the analytical screening gradient (Figure 3f) and NOT from the analytical focused gradients of this intermediate step. This would enable direct transfer from the analytical screening gradient to the preparative purification using a focused gradient.
Based on the gradient slope for the analytical separation and the retention time for each peak of interest, the modifier concentration at which each peak eluted was calculated; 14% modifier for the intermediate and 29% modifier for the product. Based on those calculations, 2-minute focused gradients were developed centered on those percentages, with starting and ending modifier concentrations that were 5% lower and higher, respectively, than the target percentages. For the synthesis intermediate, a gradient from 9-19% was used, while for the product, a gradient from 24-34% was used. All other analytical conditions were kept constant. The resulting chromatography for the intermediate [I] and product [P] using their respective focused gradients are shown in Figure 5, along with the results for the original screening gradient.
![Evaluation of the intermediate [I] and product [P] by UPC2 on an ACQUITY UPC2 BEH 2-EP, 1.7 μm, 2.1 x 50 mm Column.](/content/dam/waters/en/app-notes/2014/720005064/720005064en-f5.jpg.82.resize/img.jpg)
These results seem to support the use of focused gradients for SFC, and are extended next to preparative separations. It is important to demonstrate the ability to move from the analytical screening directly to the focused preparative separation to maximize utility and time savings. Figure 6 shows the preparative separation of the intermediate (top) and product (bottom) with their respective focused gradients. Mass directed fraction collection was used to collect the fractions of interest for multiple injections of each sample mixture.
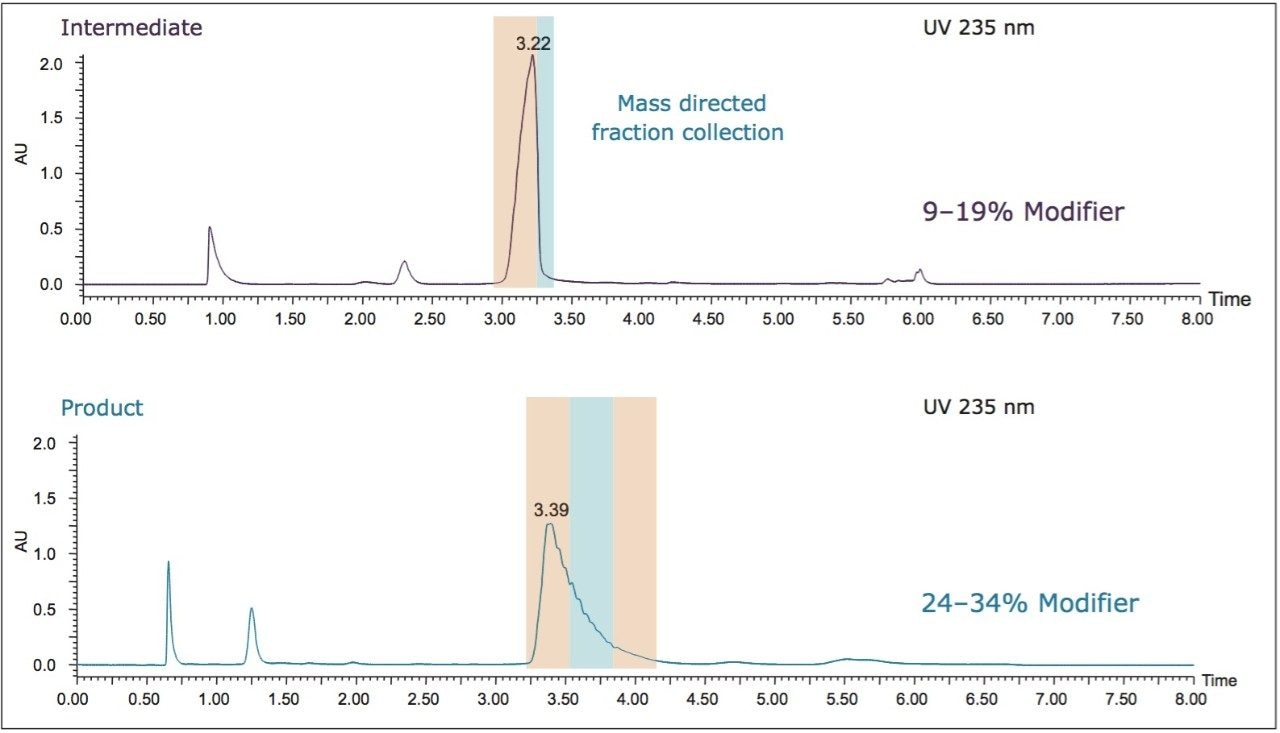
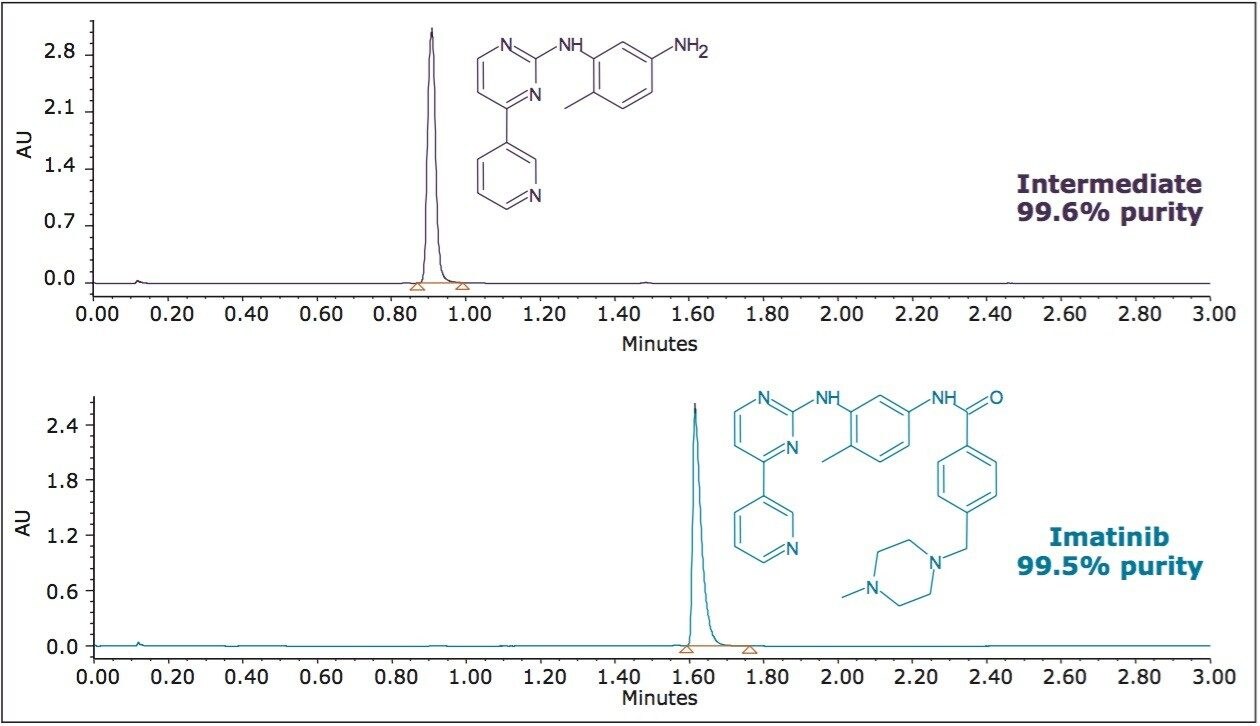
The collected fractions for each analyte were pooled prior to analysis using the original screening gradient on the UPC2 System to confirm purity. For each analyte, approximately 120 mg was collected with purities in excess of 99% (shown in Figure 7).
This example highlights the total workflow potential for purification, with the ability to do fast method development at the analytical scale with subsequent scale-up directly to a preparative purification using a targeted focused gradient. The initial method from UPC2 can then be used for final purity confirmation of the purified product. In this example, the combination of column dimensions (matched for L/dp) and flow rates yielded similar density profiles for the analytical and preparative separations, enabling successful method transfer without the need for additional density modulation.
Because of the unstable nature of the chiral compound analyzed in this case study, typical reversed-phase chromatographic methods could not be used. For the same reason, normal method development tools in UPC,2 like modifier or additive variation, were not available for exploration. The method was limited to acetonitrile as the modifier, in which the compound showed the greatest stability. Method development was therefore limited to evaluation of the effects of column chemistry, temperature, pressure, and flow rate. The column selection was chosen based on screening of several commercially available chiral columns. Once an acceptable method was achieved under analytical conditions using the UPC2 system, parameters were scaled up for purification while maintaining L/dp. Unlike the scale-up described in the case study for Imatinib, this particular combination of flow rates and column dimensions did not yield similar average density profiles for the analytical and preparative separations. The density simulations were employed to further guide the scale-up to preparative conditions, modulating the density of the preparative scale separation to approximate the same average density calculated for the analytical separation. The resulting chromatography and density calculations are shown below in Figure 8.
It is important to note that the density calculations for this exercise, using the NIST REFPROP database, are based on the physical properties of methanol, rather than acetonitrile. This is because properties of CO2 /acetonitrile binary mixtures are not available from NIST or from any other authentic sources. However, the densities of the two solvents are comparable (methanol = 791.8 g/mL and acetonitrile = 786.0 g/mL at normal temperature and pressure). The intent of the density modulation approach is to minimize the DIFFERENCES between the average densities of the two systems, and does NOT depend on the ABSOLUTE density values of any of the systems. This means that, if we can assume a linear variation of the density values of the CO2 /acetonitrile mixture from the CO2/methanol mixture, calculation of the difference between the average density values, using CO2/methanol properties, should not be very different from calculations based on CO2 /acetonitrile properties. Even with this caveat, this example again highlights the ability to achieve predictable scaled separations, based on SFC methods developed at the analytical scale using UPC.2
The application of a systematic approach using density modulation for scaling facilitates the efficient, predictable transfer of SFC methods from the analytical to the preparative purification scale. In some cases, a cognizant choice of column dimensions and flow rates may be a logical first step before applying the density modulation strategy. In other cases, as in the Imatinib case study presented here, where column dimensions are based on column availability and maintaining L/dp, and flow rates are based on optimum instrumental parameters, density profiles may be inherently matched, requiring no additional density modulation. In any case, density simulations are a valuable tool in understanding the nature of the separations, and can be used to guide additional density modulation by adjustments to the ABPR, to yield similar average density profiles between the analytical and preparative scale separations. In addition, as are frequently used for LC applications, focused gradients can be used as an effective tool for preparative SFC purification. The ability to transition directly from an analytical scale screening separation on UPC2 to a preparative scale purification with a focused gradient and mobile phase density modulation represents a significant savings in time and costs associated with method development for preparative SFC purifications.
720005064, May 2014