The PATROL UPLC Process Analysis System brings reference-standard methodology to process development and is directly scalable through commercial operations, eliminating the need to calibrate spectroscopic sensors or to send suspect samples to an offline QC laboratory. This application note discusses the use of UPLC for online reaction monitoring of in-process manufacturing samples.
Better throughput, yield, and process understanding are possible with the use of PATROL UPLC Process Analysis System for in-process reaction monitoring. It can provide improved reliable detailed information about the presence and amounts of both the desired product and low level product and process impurities in one run, when compared with other typical PAT sensors.
For pharmaceutical and biopharmaceutical companies, among other industries, Process Analytical Technology (PAT) is a critical component of the overall manufacturing process. It is relied upon to provide richer process understanding, consistent product quality at maximum yields, and with minimal waste.
PAT involves taking timely measurements throughout the production process to verify the quality of in-process batches and to understand performance in each of the critical steps of that process. Many different sensor technologies are employed throughout the manufacturing process to measure the attributes of the in-process batches. Deployment of appropriate sensors to monitor identified critical quality attributes (CQAs) can aid in maintaining process control and functioning well within the established design space of the process.
Typically, process steps such as reaction monitoring are assessed by spectroscopic sensors, which include near-infrared spectroscopy (NIR) or Raman spectroscopy. These techniques have the ability to provide real-time information about the reaction progression but lack the ability to effectively resolve and quantify multiple components in a sample, particularly if the concentrations of some of the components are at low levels.
Performance of these sensors needs to be benchmarked against a reference standard, which in most instances is high performance liquid chromatography (HPLC) because it is a more selective and sensitive technique, with a broader linear dynamic range, and has the ability to quantify multiple components within complex samples, including low level impurities.
HPLC is the most widely-used technique in pharmaceutical QC laboratories. However, its long run times and complex system operation have prevented it from being routinely used for atline or online analysis.
With the introduction of Waters UltraPerformance LC (UPLC) technology, it is now possible to achieve near real-time chromatographic analysis for in-process samples. UPLC is delivered in a system that includes integrated hardware and software, offering a simple design that requires little to no user input.
The PATROL UPLC Process Analysis System brings reference-standard methodology to process development and is directly scalable through commercial operations, eliminating the need to calibrate spectroscopic sensors or to send suspect samples to an offline QC laboratory. This application note discusses the use of UPLC for online reaction monitoring of in-process manufacturing samples.
In this study, the conversion of acetylsalicylic acid (ASA) to salicylic acid was monitored. One liter of ASA was prepared at 0.3 g/L in water. The repeatability of the system was assessed prior to the reaction initiation by drawing from the vessel at ambient temperature. The vessel was then placed in a 75 °C heated bath and 10 mL of nitric acid added. The reaction was sampled and analyzed under the following conditions:
|
Column: |
Waters ACQUITY UPLC HSS T3 2.1 mm x 50 mm, 1.8 μm |
|
Eluents: |
A: 0.1% Formic acid in water B: 0.1% Formic acid in acetonitrile |
|
Gradient: |
5% to 80% over 2 minutes; Curve 6 |
|
Flow rate: |
0.8 mL/min |
|
Temp.: |
50 °C |
|
Inj. Volume: |
1 μL |
|
Detection: |
243 nm; 40 Hz; Time Constant 0.025 sec |
|
Wash: |
70:15:15 Acetonitrile/water/ isopropanol |
|
Purge: |
1 mL (4x volume of transfer line) |
|
Run time: |
2.5 min |
|
Cycle time: |
4 min, 10 sec |
UPLC is based upon the use of sub-2-μm column particles and system technology that takes advantage of the benefits of these particles. Since its introduction, many users have transferred their HPLC QC methods to UPLC with great success, realizing tremendous improvements in sensitivity, throughput, and resolution.
The PATROL UPLC Process Analysis System brings these significant improvements to the manufacturing floor in a manner that allows LC to be used as a real-time sensor. It is a holistically-designed system that integrates UPLC technology, control software, and a ruggedly-engineered sample management module for managing the samples and workflow in an in-process environment. The system’s components, along with all solvents, waste, and standards, are contained within an enclosed case that is compatible with all requirements of a manufacturing environment.
The system is designed to be compatible with both online (direct automatic sampling from a process stream) and atline (normal sample drawn from a process stream) analysis. The ACQUITY UPLC Process Sample Manager (PSM) can be interfaced with process streams or reactors to provide real-time analysis and quantification without the need for user intervention. Data can be sent to Distributed Control Systems (DCS) or LIMS for completely automated monitoring. For atline applications, the system features a walk-up interface with barcode scanning capabilities that eliminates the need for information input from the technician. It also provides sample chain of custody with the 21CFR Part 11 compliant-ready Empower Software.
The ability to quantitate all of the components in a reactor during a synthesis reaction results in the highest conversion to the target compound with the lowest generation of side products. The real-time monitoring capabilities of the PATROL UPLC Process Analysis System maps out the amounts of all the components in the reactor to determine the optimal time to quench the reaction and forward process.
To assess system suitability prior to reaction initiation the repeatability of the transfer of a separation of the starting material was determined. The %RSD for the transfer and analysis of the starting materials was determined to be well within method requirements and is listed in Table 1. Once the reaction was initiated, the aliquots from the reaction vessel were automatically transferred to the PATROL UPLC Process Analysis System and the peak area % of each component present was monitored.

As the reaction progressed, the system was able to separate all the components with good resolution and mapping the reaction progression. Selected injections throughout the reaction are displayed in Figure 1 to demonstrate the ability to monitor reaction progression by assessing the amounts of each component within very short time intervals throughout the reaction.
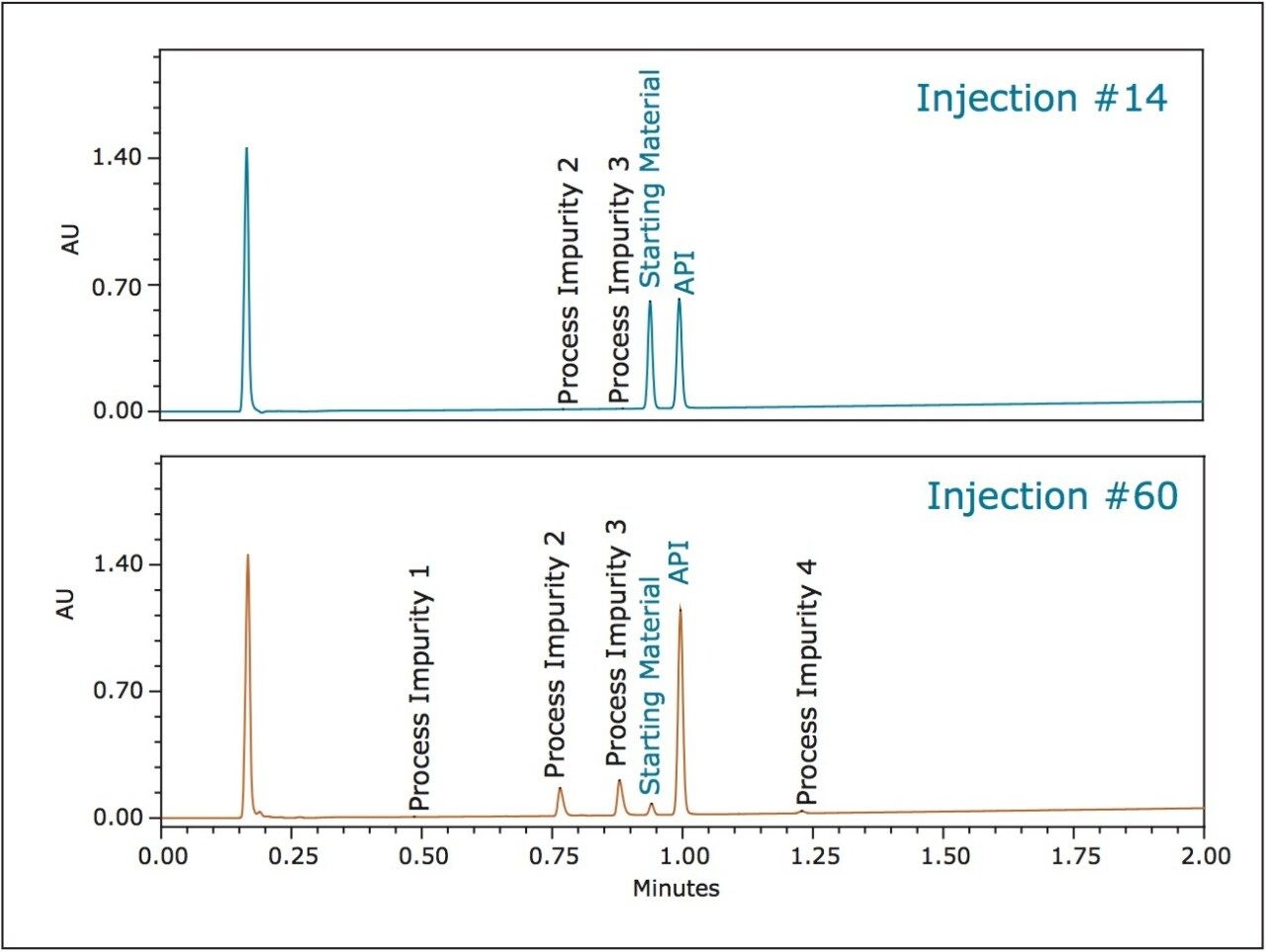
The PATROL UPLC Process Analysis System has a large linear dynamic range which allows for the simultaneous quantification of both high- and low-level components within the same chromatographic run (Figure 2), even at levels below 0.05% of the major components (Table 2).
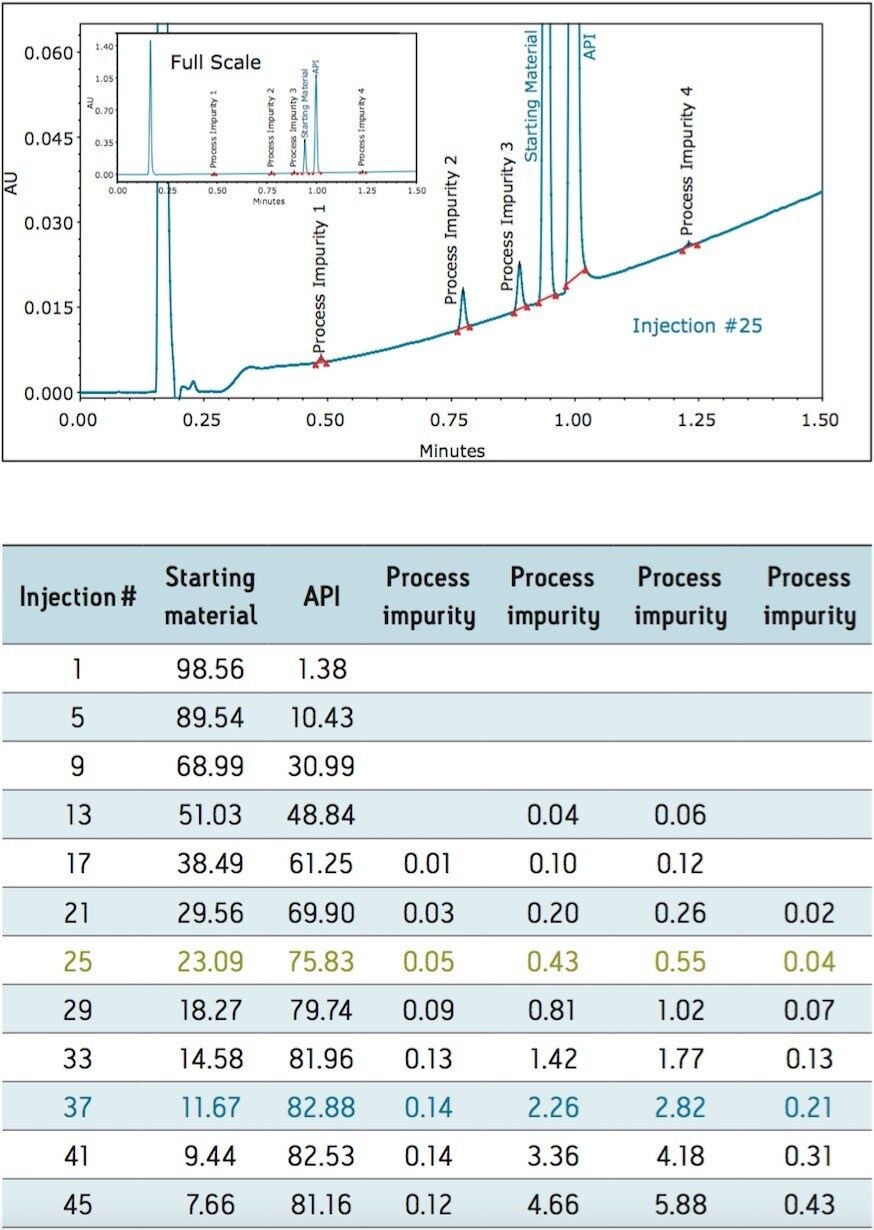
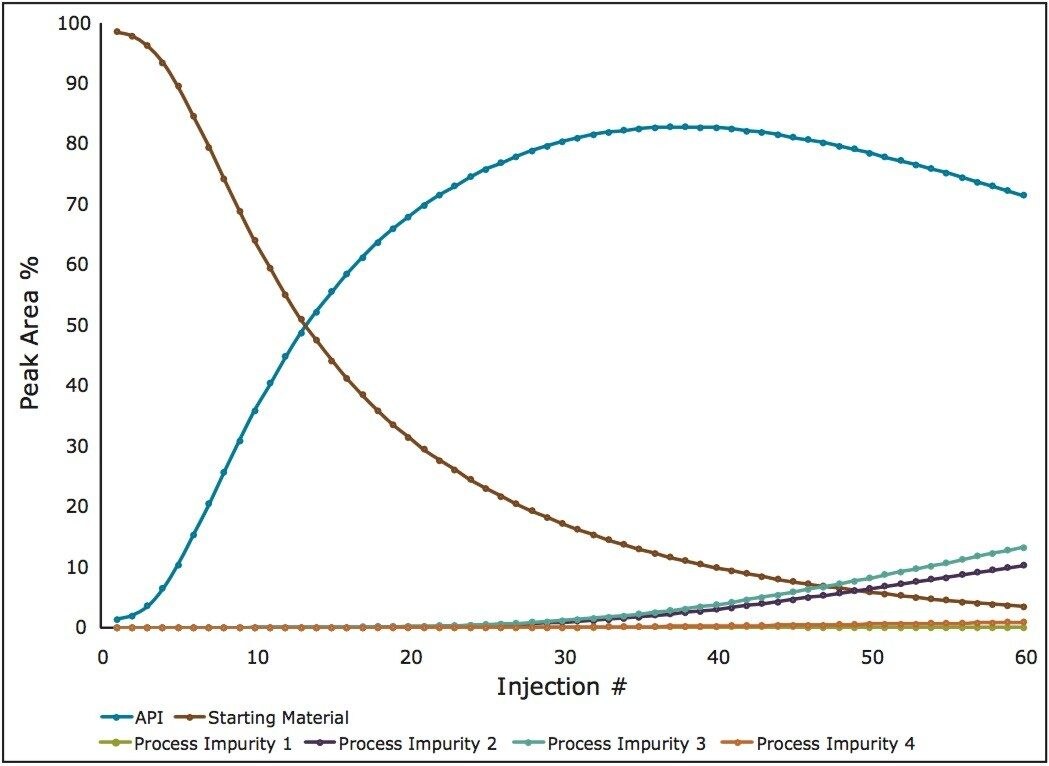
Online spectroscopic sensors cannot simultaneously quantify such a complex matrix with such varying concentrations. By plotting the %Area of each of the components from the chromatogram a map of the reaction can be generated (Figure 3).
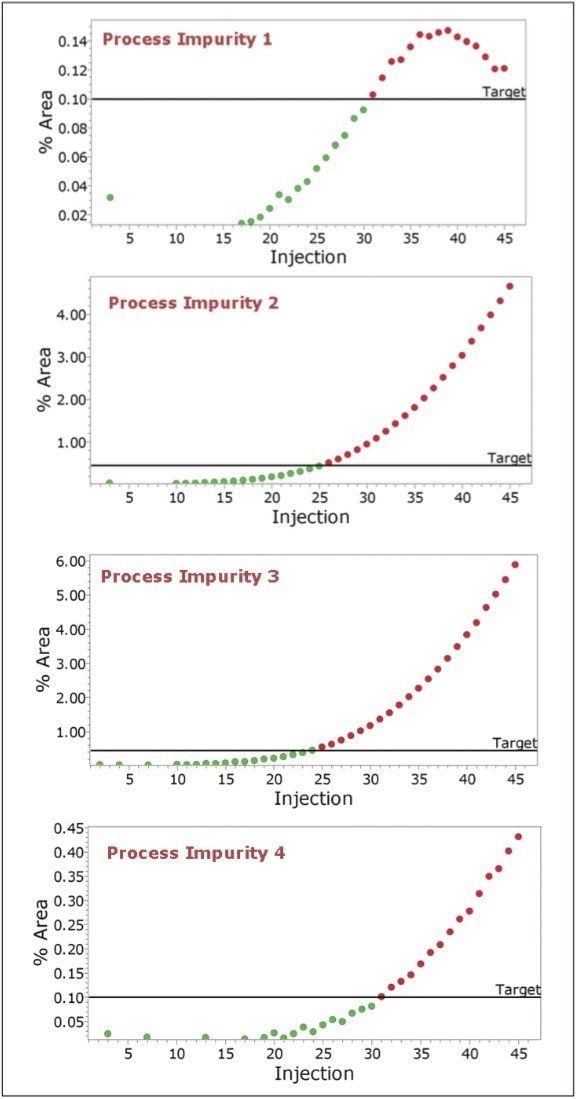
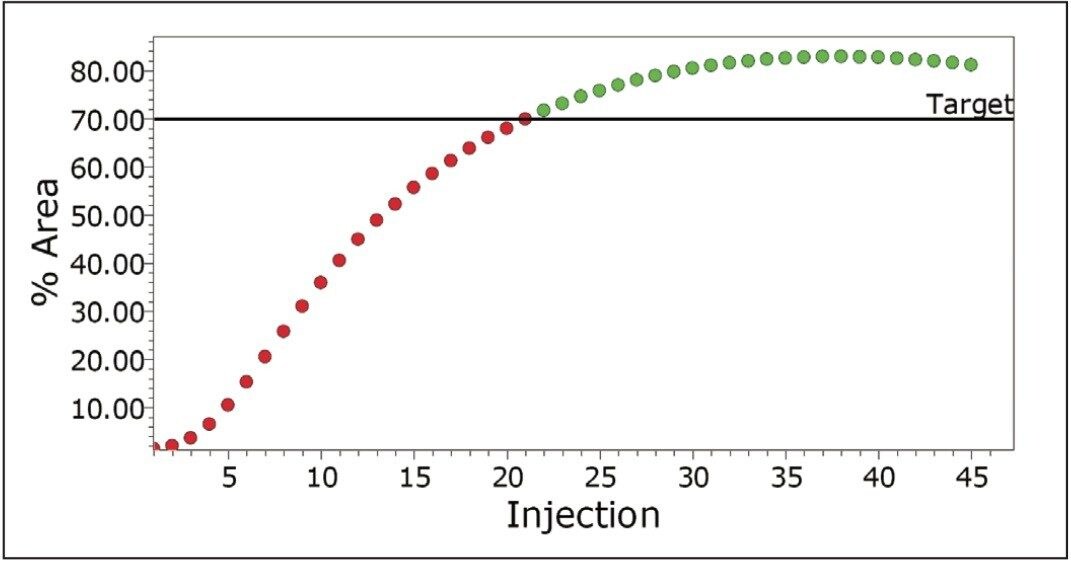
Empower Software incorporates powerful processing and reporting capabilities which allow the user to define target levels for each of the components in the reactor. The system can flag the point at which impurities reach a critical level (Figure 4) and/or the point when the API reaches a target level (Figure 5).
To learn more: www.waters.com/patrol
720002605, May 2014