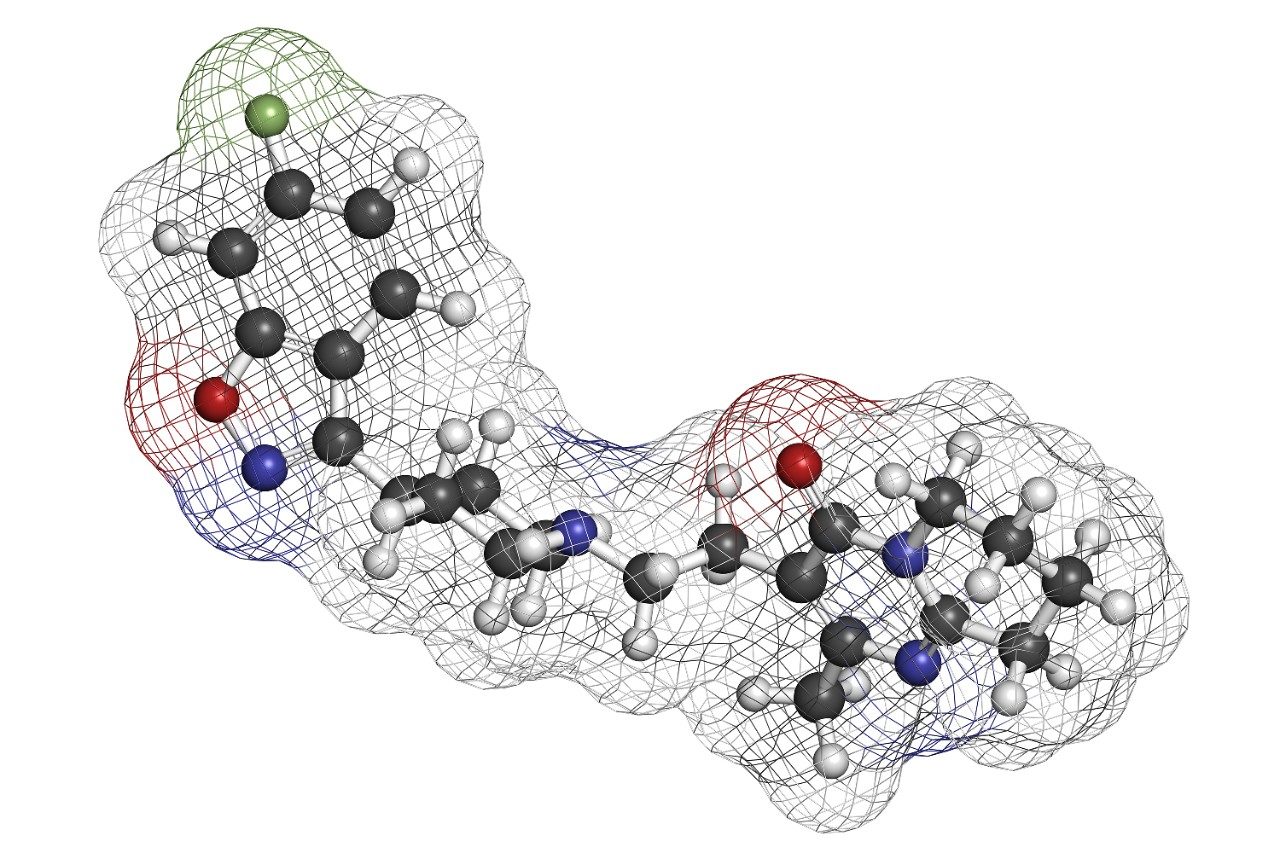
In this study, whole blood samples containing risperidone, its hydroxylated metabolite, 9-OH risperidone, and the internal standard, clozapine were extracted in both the traditional manner and in-well using a 96-well Ostro sample preparation plate.
Dried blood spot (DBS) analysis has been rapidly gaining momentum in the pharmaceutical industry. Economic and ethical issues surrounding laboratory animal usage and cost associated with shipping and storing biological samples has made DBS analysis an attractive option. DBS analysis can generate high analyte recoveries. However, this technique does little to eliminate endogenous interferences. Interferences, in particular residual phospholipids (PLs), are a major source of concern in bioanalysis. PLs build up in LC-MS/MS systems and are one of the major causes of matrix effects. Amongst other problems, matrix effects alter mass spectrometry response in an unpredictable manner, decrease method robustness, and add to method variability. The Ostro Pass-through 96-well Sample Preparation Plate can be used to eliminate greater than 99% of residual PLs. The Ostro Pass-through 96-well Plate further simplifies the DBS extraction process through the use of 96-well format plates which simultaneously extract analytes, reduce PLs and filter out the spot. DBS punches can be extracted in-well, diluted, and directly injected onto an LC-MS/MS system. This novel method significantly reduces sample preparation time and eliminates potential analyte losses due to extract transfer, dry down, and reconstitution. In this work, whole blood samples containing risperidone, its hydroxylated metabolite, 9-OH risperidone, and the internal standard, clozapine (Figure 1) were extracted in both the traditional manner and in-well using the Ostro Pass-through 96-well Plate.
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Mobile phase A |
0.1% NH4OH in H2O |
|
Mobile phase B |
50/50 ACN/MeOH |
|
Flow rate |
0.6 mL/min |
|
Injection volume |
40 μL |
|
Injection mode |
Partial Loop |
|
Column temp. |
35 °C |
|
Sample temp. |
15 °C |
|
Strong needle wash |
60:40 ACN: IPA + 2% HCOOH |
|
Weak needle wash |
95/5 H2O/MeOH |
|
Time(min) |
Profile |
Curve |
|
|
%A |
%B |
||
|
0.0 |
75 |
25 |
6 |
|
2.0 |
0.5 |
99.5 |
6 |
|
3.0 |
0.5 |
99.5 |
6 |
|
3.1 |
75 |
25 |
6 |
|
3.5 |
75 |
25 |
6 |
|
Capillary voltage: |
3.0 V |
|
Desolvation temp.: |
550 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
1000 L/Hr |
|
Collision cell pressure: |
2.6 x 10(-3) mbar |
|
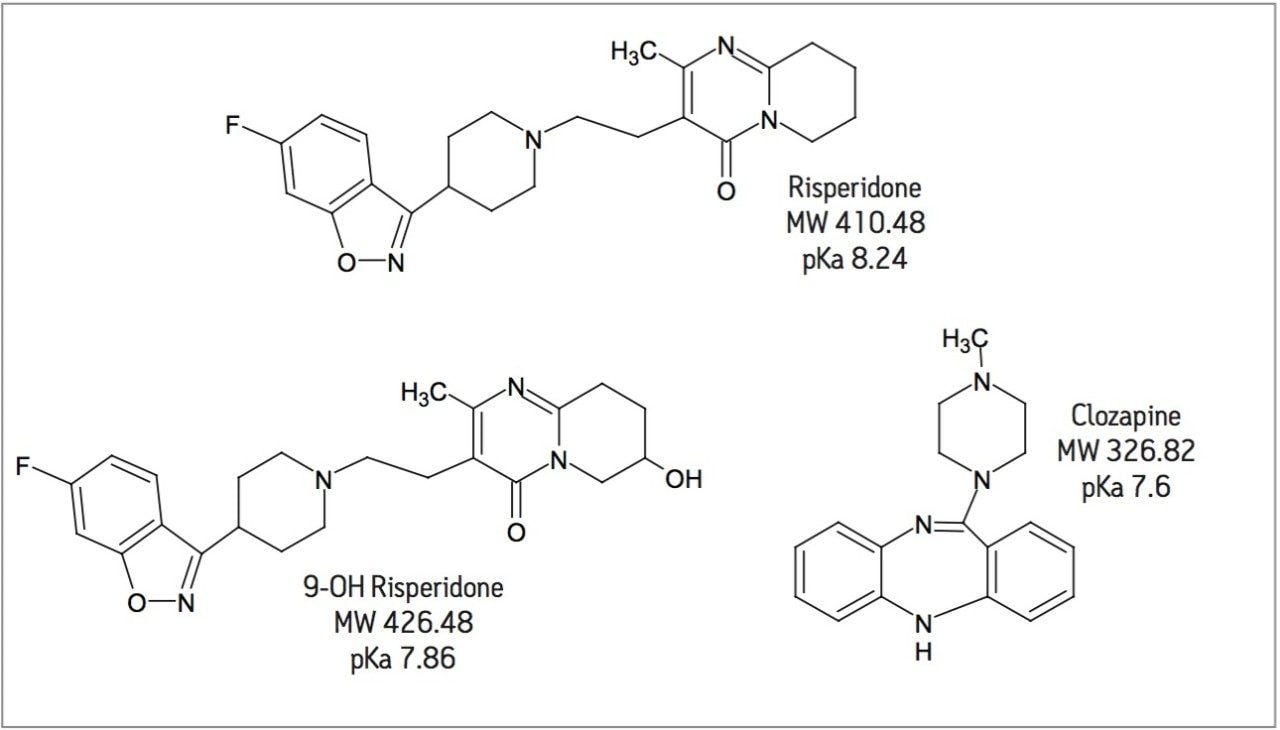
MRM transition monitored, ESI+: |
See Table 1 |
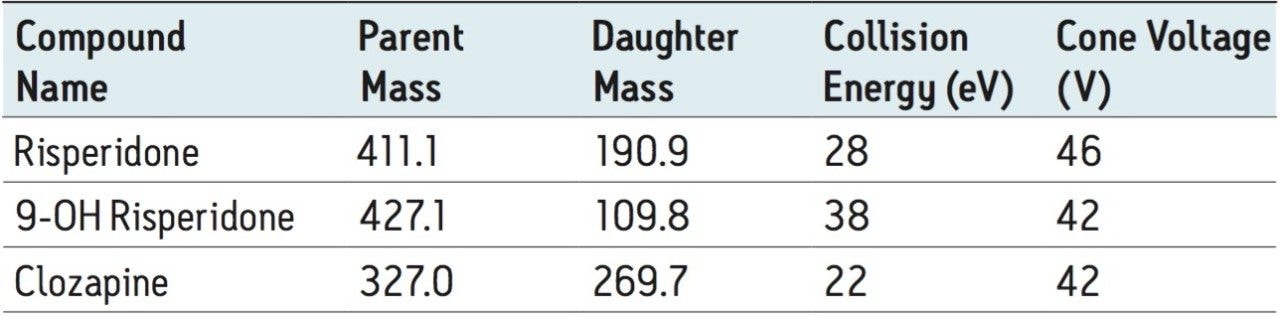
Whole blood samples were spotted in 20 μL aliquots onto Whatman DMPK-C untreated cards. The samples were allowed to dry for 2 hours at room temperature. Three-mm punches were taken using the Harris 3 mm micropunch and were placed in individual 1.5 mL Eppendorf tubes for traditional DBS extraction or directly into the wells of the Ostro Pass-through 96-well Plate. The traditional extraction was performed using 250 μL of 5% water in methanol in 1.5 mL Eppendorf tubes, followed by vortexing for 1 minute, centrifuging for 1 minute at 3000 g, and finally transferring the extract to a 96-well collection plate. Extraction using Ostro Pass-through 96-well Plate was performed as a single-step in-well extraction. Three-mm dried blood spots were added into the wells of the Ostro 96-well plate (Figure 2), 250 μL of 5% water in methanol was used as the extraction solvent, the plate was vortexed for 1 minute and vacuum was applied for 5 minutes. Eluates from both techniques were diluted with 250 μL of water prior to direct injection on the LC-MS/MS system.
MS was performed in positive ion mode. As direct injection of DBS extracts typically results in very dilute samples, the Xevo TQ-S was used to significantly increase sensitivity. This facilitated the ability to directly inject the samples and skip potentially problematic evaporation and reconstitution steps.
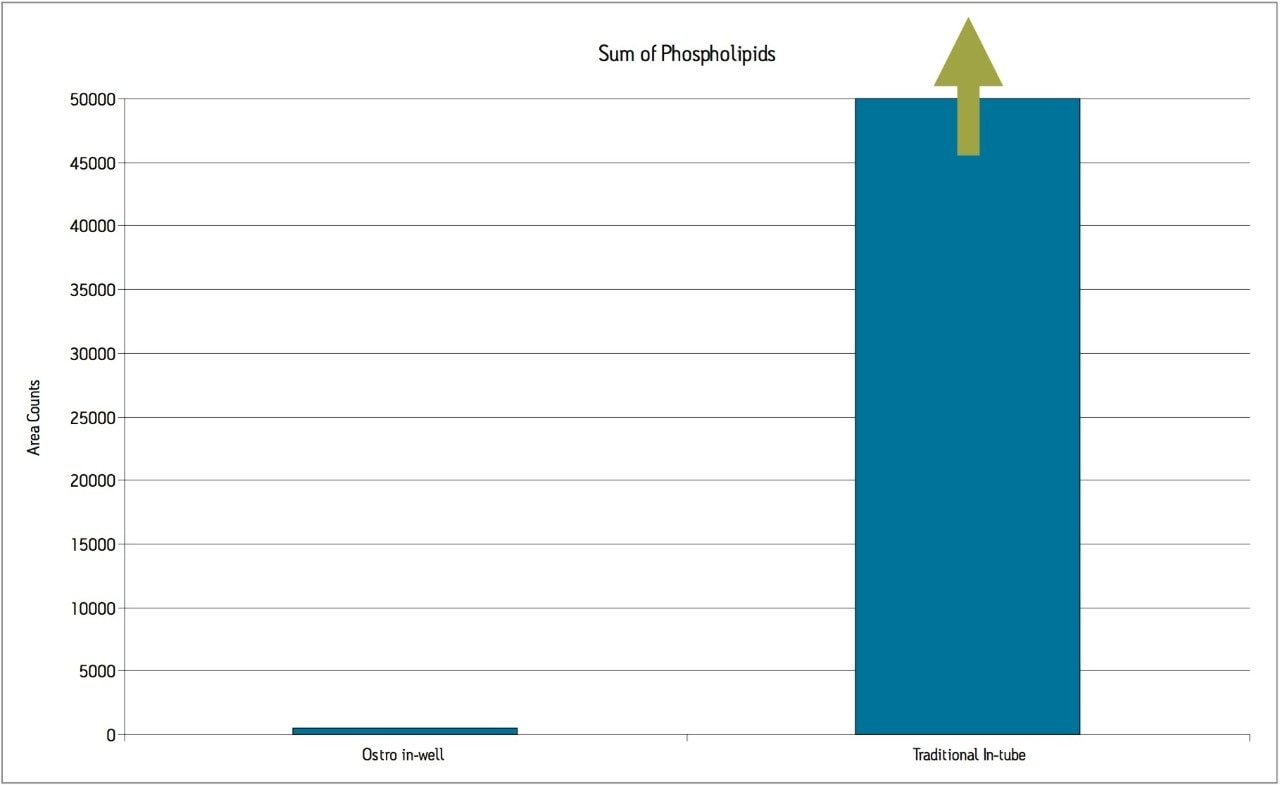
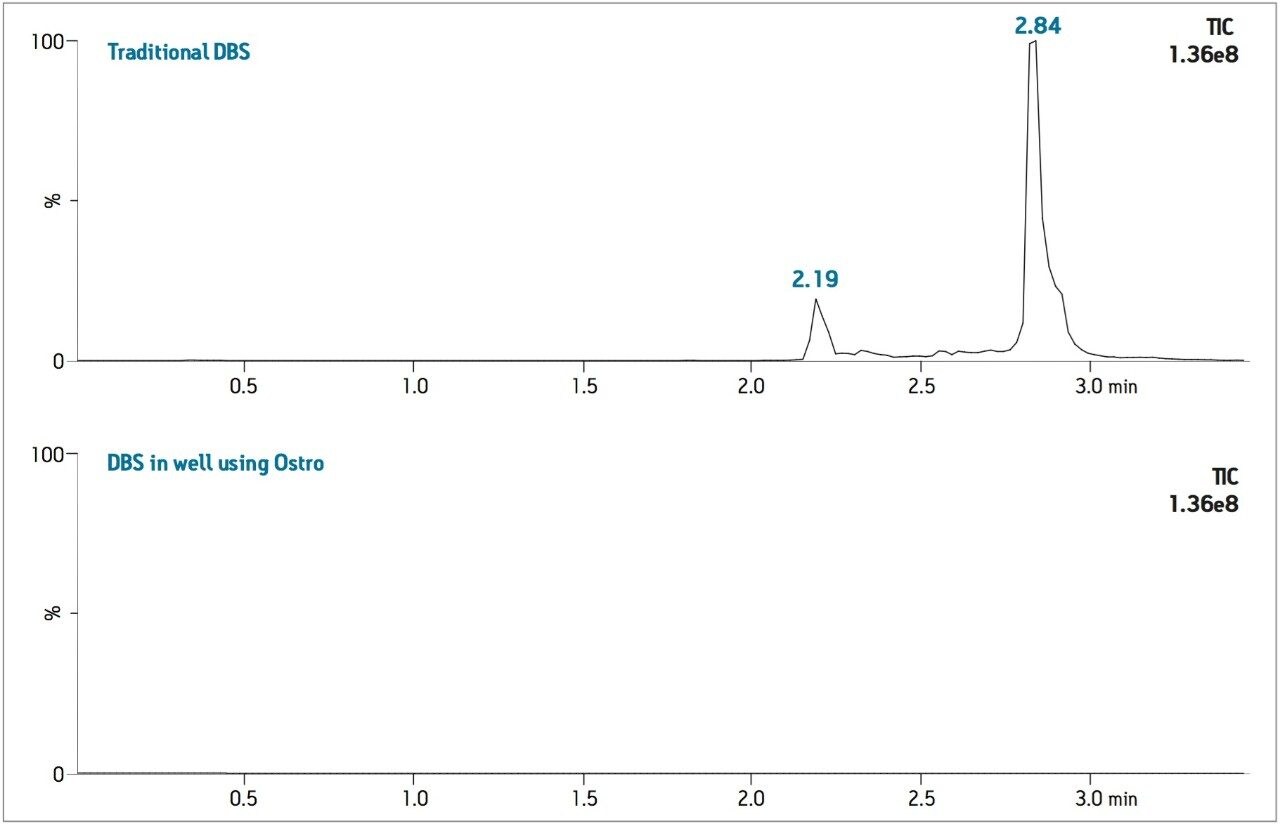
The levels of PLs remaining after each sample preparation technique were compared. To compare PL levels, five PLs were quantified from DBS punches extracted in single tubes versus the DBS punches extracted in-well using the Ostro PLR plate. The Ostro Pass-through Plate removed 99.9% of the phospholipids relative to the traditional DBS method (Figure 3). To visually demonstrate remaining residual phospholipids, the total ion current (TIC) of 5 individual phospholipids is shown for both traditional DBS extraction and DBS extraction in well using Ostro (Figure 4). When the Ostro Pass-through Plate is used, the PL levels are negligible and no build-up occurs. When traditional DBS is used, a significant amount of PLs are present and accumulate throughout the run despite the 1-minute hold at a high percentage of organic solvent meant to prevent PL build-up (Figure 5).
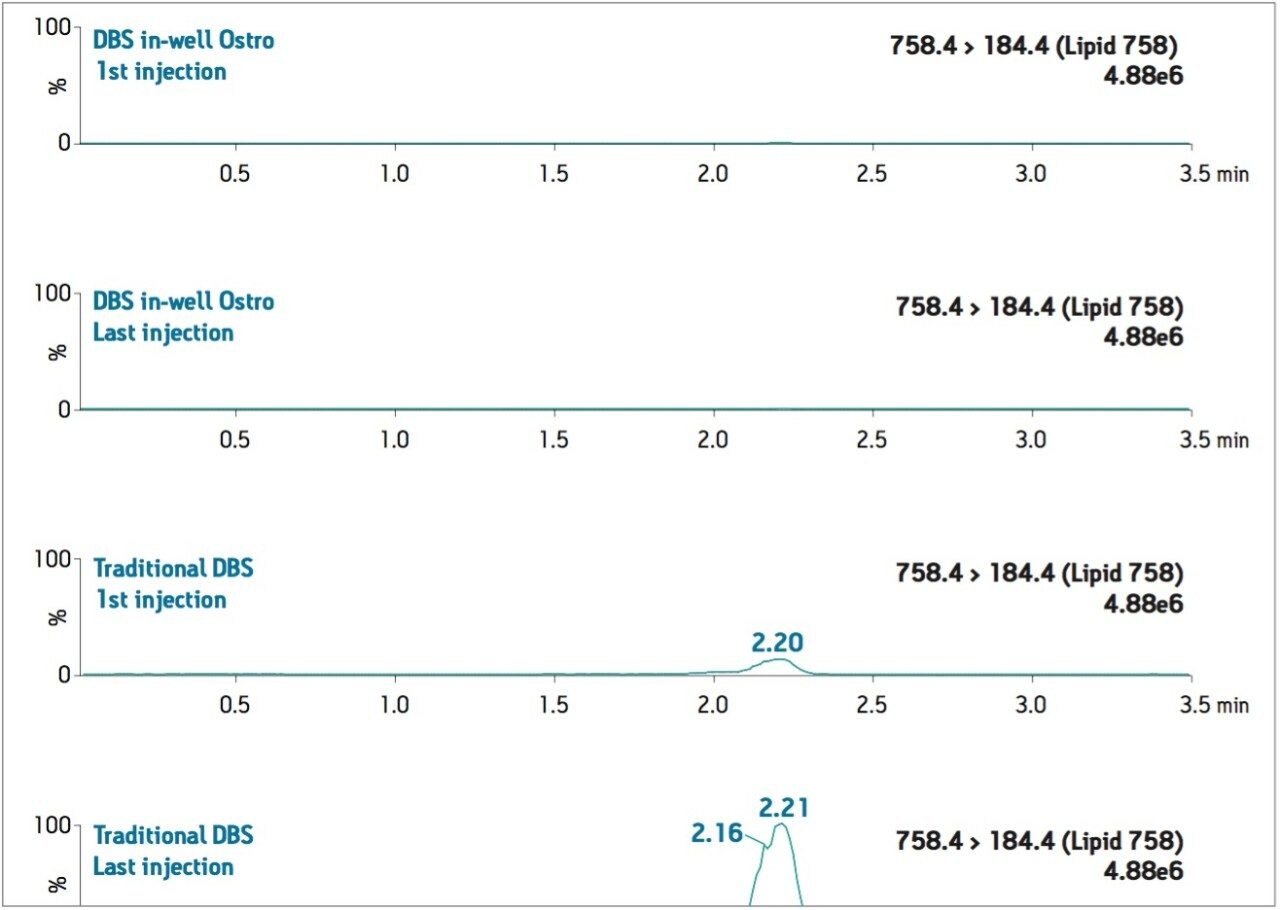
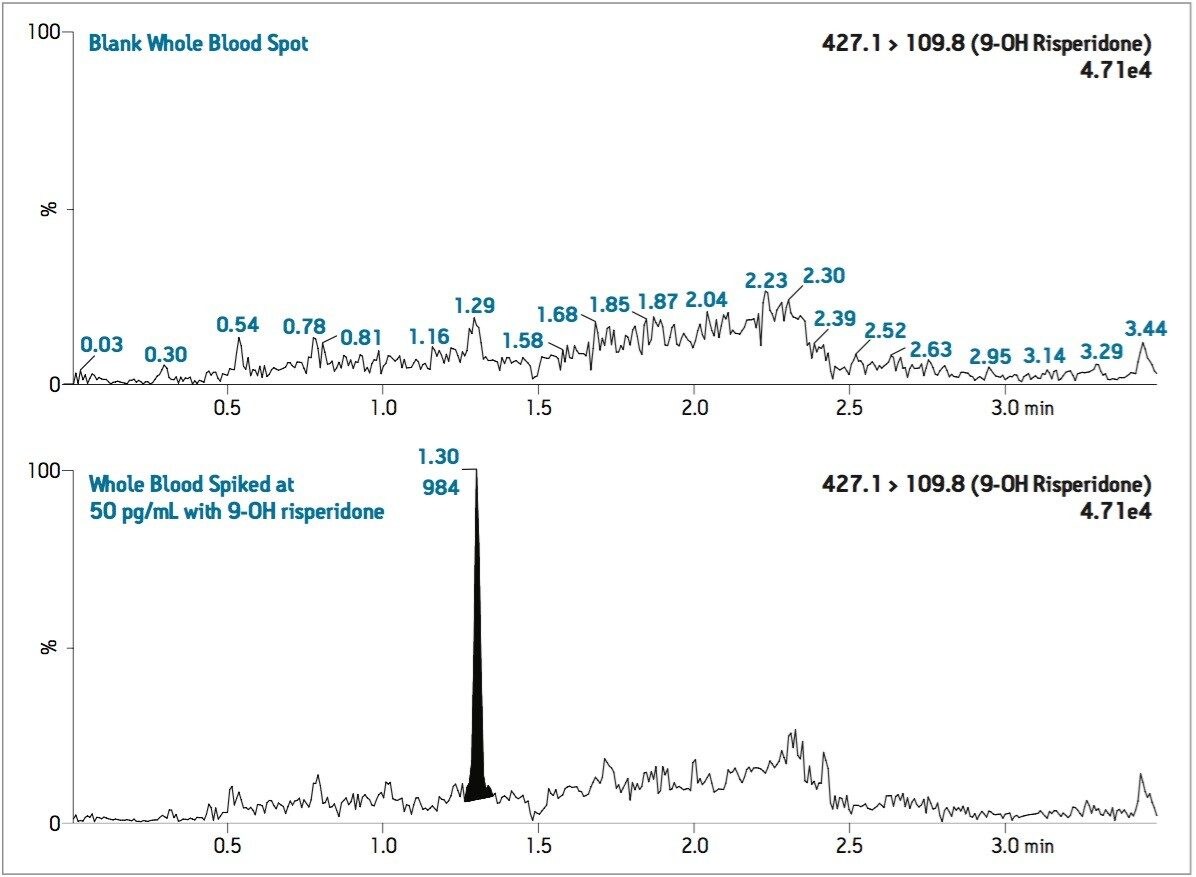
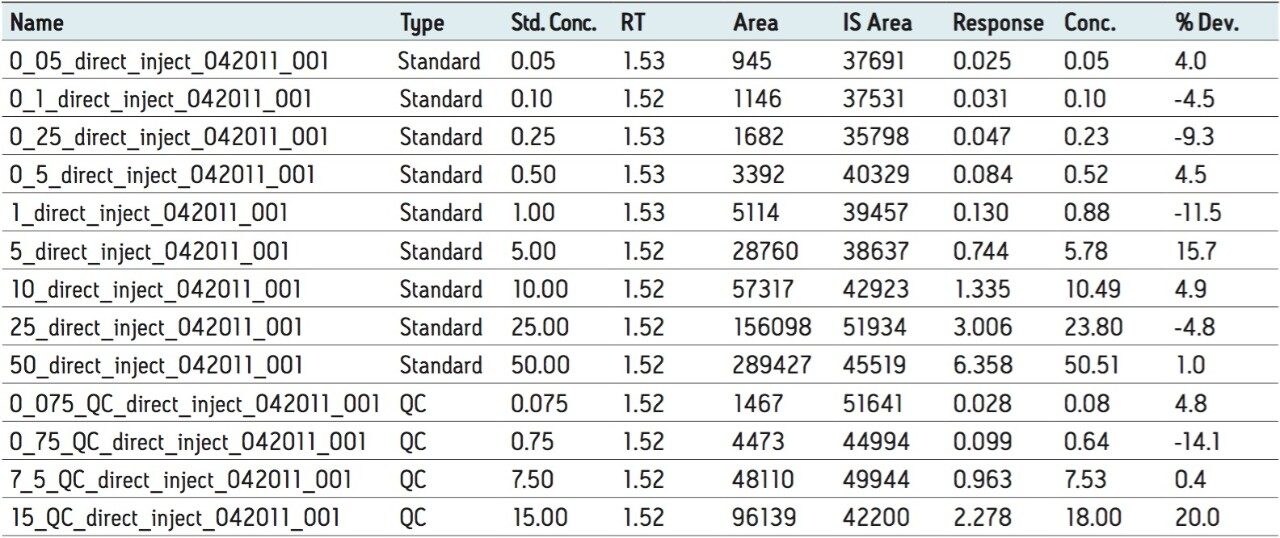
Semi-validation was performed, and calculated QC sample concentrations were within 15% of expected, meeting regulatory criteria. LLOQ’s of 0.05 ng/mL for 9-OH risperidone (Figure 6) and the parent compound were achieved in whole blood. Standard curves were linear over 3 orders of magnitude, from 0.05 ng/mL to 50 ng/mL in whole blood (Figure 7).
720004047, October 2014