
This is an Application Brief and does not contain a detailed Experimental section.
To transfer the USP method for topiramate, which uses refractive index detection, from HPLC to UPLC. The successful transfer will reduce analysis time, improve data quality, and increase sample throughput in the manufacturing, quality-control laboratory.
The majority of today’s compendial methods are considered dated and do not take advantage of the recent advances in today’s instrumentation and stationary phases. The HPLC methods listed in the United States Pharmacopeia (USP) monographs are typically long and consume a high volume of solvents. With the evolution of new LC technology, many companies want to migrate to UPLC technology with sub-2-µm particle columns to reduce analysis time and solvent usage, to improve chromatographic performance, and to maintain or improve data quality. Adopting UPLC technology streamlines laboratory processes, improving efficiency, productivity, and profitability of pharmaceutical manufacturing facilities. Transferring methods to UPLC that require refractive index detection has been challenging due to the lack of availability of a low dispersion option that is compatible with UPLC peak widths. The Waters ACQUITY Refractive Index (RI) Detector empowers laboratories that utilize HPLC methods with refractive index detection to take advantage of the benefits made possible with the introduction of UPLC technology.
Topiramate is a prescription medication used to prevent and control seizures. In this study, a compendial HPLC method for topiramate assay written in the USP was transferred from HPLC to UPLC. Performance of the UPLC method was measured by evaluating the system suitability of the standard solution against the requirements listed in the USP monograph for topiramate drug substance.
The standard solution used in this study was prepared according to the assay method defined in the USP monograph for topiramate drug substance. The USP method was run accordingly on an Alliance HPLC System equipped with 2414 Refractive Index (RI) Detector and an appropriately selected Waters column guided by the Waters Reversed-Phase Selectivity Chart. The USP designates an L1 packing for the assay method and suggests using a Intertsil ODS-3 column.
Using the Reversed-Phase Selectivity Chart, a Waters XSelect HSS C18, 4.6 x 250 mm, 5.0-µm Column was chosen for testing. The compendial method was transferred to UPLC using the Waters UPLC Columns Calculator (as described in our application note 720003721EN). A detailed description of the HPLC and UPLC instrument conditions is listed in Table 1.
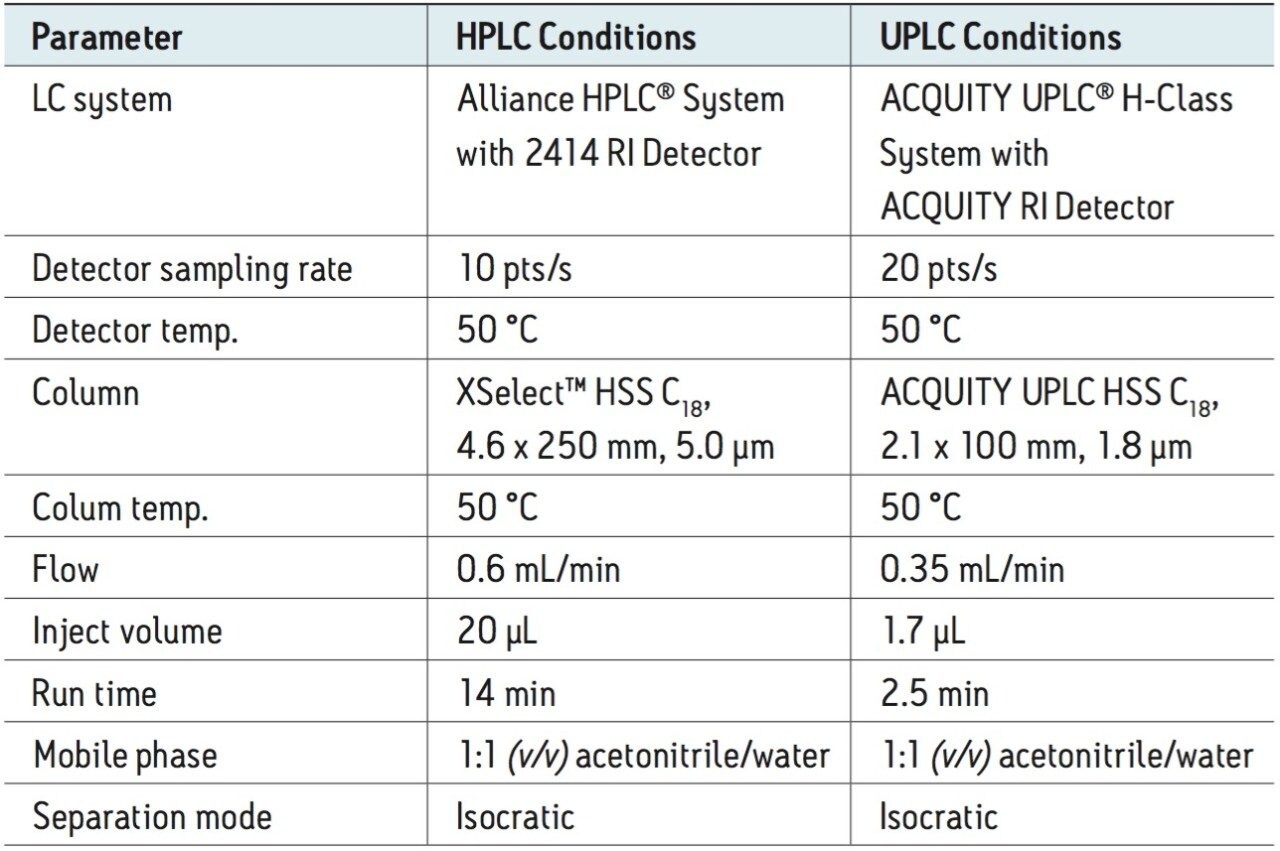
The UPLC method was run on an ACQUITY UPLC H-Class System equipped with the ACQUITY RI Detector, producing narrower peaks and reduced analysis time. The ACQUITY RI Detector is optimized for UPLC separations, with a low internal volume that delivers reduced dispersion but without sacrificing baseline stability. A comparison of the topiramate standard solution acquired using the Alliance HPLC and ACQUITY UPLC systems is displayed in Figure 1. By transferring the HPLC method to UPLC, we reduced analysis time from 14 to 2.5 minutes, which represents an overall time savings of 82%.
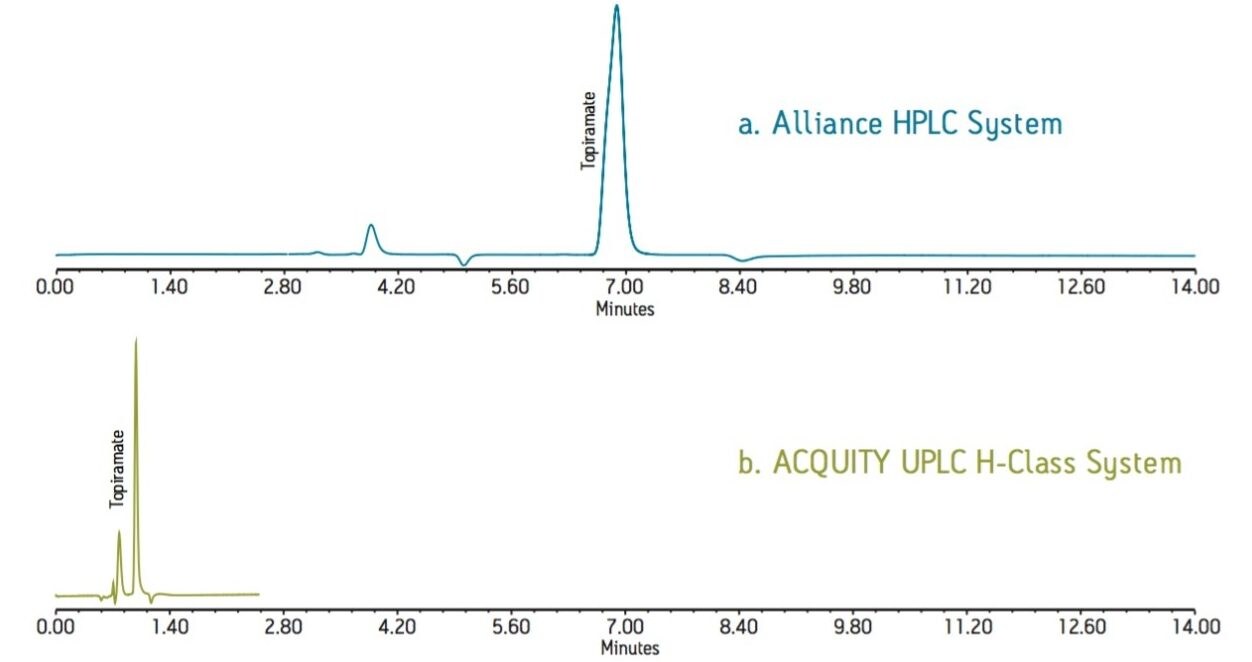
Figure 1. Topiramate standard solution
a. Alliance HPLC system with a XSelect HSS, C18 4.6 x 250 mm, 5.0-µm Column
b. ACQUITY UPLC H-Class System with an ACQUITY UPLC, HSS C18, 2.1 x 100 mm, 1.8-µm Column
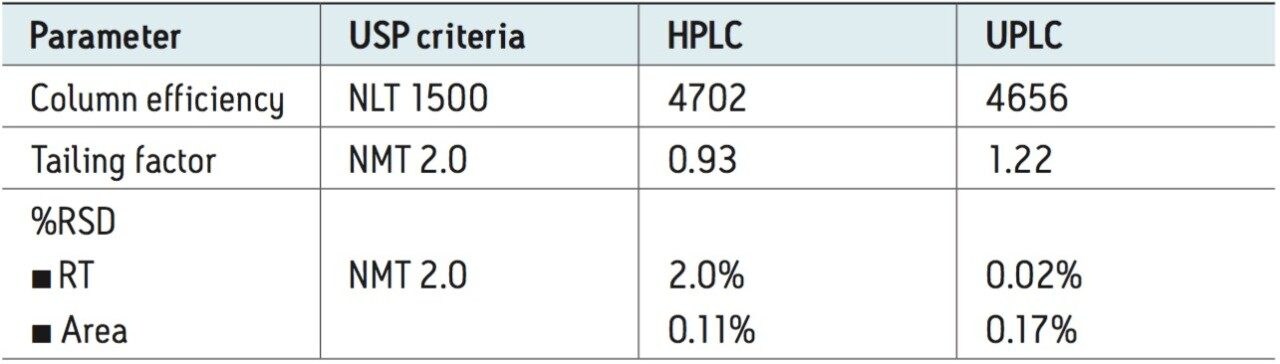
Performance of the methods was verified by comparing the system suitability results against the requirements defined in the USP monograph for topiramate drug substance. System suitability results, HPLC and UPLC, for the five replicate injections of the standard solution passed the USP requirements for the assay method as summarized in Table 1. The retention time and area repeatability of the UPLC method were substantially lower than the USP criteria and comparable to the HPLC results. The efficiency of the ACQUITY UPLC Column was well above the USP requirements. The excellent performance of the UPLC method and reduction in analysis time are essential elements to minimize downtime and enhance throughput for routine analysis of pharmaceutical products.
The HPLC compendial method for topiramate was successfully transferred to the ACQUITY UPLC H-Class System equipped with the ACQUITY RI Detector. The ACQUITY RI Detector was designed with reduced internal volumes and the advanced detection technology required for detection and accurate definition of the narrow peaks generated in a UPLC separation. It extends UPLC technology to the LC applications that are dependent upon refractive index detection. The UPLC/RI method provided an 82% reduction in runtime while meeting the USP requirements for system suitability. The amount of mobile phase per UPLC injection equals 0.88 mL, compared to 8.4 mL for the HPLC method, which represents a 90% savings in mobile phase consumption. Overall, the resulting UPLC method provides cost savings for solvent and waste disposal. Implementing UPLC technology provides improvements in laboratory throughput and productivity by reducing analysis time for release testing of manufacturing batches.
720004643, March 2013