
In this application, four different types of packaging material were extracted, including a high density polypropylene pill bottle (HDPE), a low density polypropylene bottle (LDPE), an ethylene vinyl-acetate plasma bag (EVA), and a polyvinyl chloride blister pack (PVC). The extracts were screened for 14 common polymer additives.
Extractables from packaging materials are a concern to manufacturers and suppliers of containers for the heavily regulated pharmaceutical and food industries.1-3 Due to these regulations, packaging material manufacturers are motivated to control and monitor their product to ensure that no potential risk exists from extractable and leachable material. Similarly, the manufacturers of supplies for industrial processes, such as plastic vessels and filters, are required to demonstrate that their products do not add any leachables in the production process.
The initial investigation, called a controlled extraction study, qualitatively and quantitatively investigates the nature of extractable profiles from critical container closure system components. It is performed early in device and packaging development. The testing involves solvent extraction techniques encompassing a range of polarity, solvent compatibility studies, and multiple analytical techniques. One of the limitations encountered in these studies involved matching the solvent extracts with the appropriate analytical technique. For example, non-polar solvent extracts can be directly injected into a gas chromatography (GC) system but must be evaporated and reconstituted with a solvent compatible with a liquid chromatography (LC) system. Likewise, water extracts must be back-extracted into a non-polar solvent for analysis by GC. UltraPerformance Convergence Chromatography (UPC2), built on the principles of supercritical fluid chromatography (SFC), allows different types of extraction solvents to be injected for separation on one system for analysis, thereby saving time and reducing sample preparation efforts.
In this application, four different types of packaging material were extracted, including a high density polypropylene pill bottle (HDPE), a low density polypropylene bottle (LDPE), an ethylene vinyl-acetate plasma bag (EVA), and a polyvinyl chloride blister pack (PVC). The extracts were screened for 14 common polymer additives. Hexane, isopropanol (IPA), and water were used as the extraction solvents. GC-MS was used to analyze hexane and IPA extracts, the ACQUITY UPLC System was used to analyze water and IPA extracts, and the ACQUITY UPC2 System was used to analyze all three solvent extracts. The UPC2 analysis was compared to the GC and UPLC chromatographic profiles.
Samples were prepared by microwave extraction. The samples of HDPE, LDPE, EVA, and PVC (2 g) were extracted in 10 mL of isopropanol or hexane for 3 h at 50 °C. Water extracts were prepared by placing 2 g of sample into 20 mL headspace vials with 10 mL of water, and keeping them in a conventional oven for 72 h at 50 °C.
|
Column: |
HP-5MS 30 m x 0.32 mm, 1.0 μm film |
|
Carrier gas: |
He at 2 mL/min |
|
Temperature program: |
35 °C for 5 min, 20 °C/min to 320 °C, hold 20.75 min |
|
Injection port: |
300 °C |
|
Injection type: |
1 μL splitless, 1 min purge |
|
Makeup gas: |
N2 at 400 mL/min |
|
Transfer line: |
350 °C |
|
Scan range: |
100 to 1500 m/z |
|
Run time: |
40 min |
|
Data management: |
MassLynx v4.1 Software |
|
UPC2 Conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 |
|
Detection: |
Photodiode Array (PDA) Detector and SQD Mass Spectrometer |
|
Column: |
ACQUITY UPC2 BEH 2-EP 3.0 x 100 mm, 1.7 μm |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
1:1 methanol/acetonitrile |
|
Flow rate: |
2.0 mL/min |
|
Gradient: |
1% B for 1 min, to 20% over 2.5 min, hold for 30 s, re-equilibrate back to 1% |
|
Column temp.: |
65 °C |
|
APBR: |
1800 psi |
|
Injection volume: |
1.0 μL |
|
Run time: |
5.1 min |
|
Wavelength: |
220 nm |
|
MS scan range: |
200 to 1200 m/z |
|
Capillary: |
3 kV |
|
Cone: |
25 V |
|
MS make-up flow: |
0.1% formic acid in methanol, 0.2 mL/min |
|
Data management: |
Empower 3 Software |
|
System: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH Phenyl 2.1 x 100 mm, 1.7 μm |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Flow rate: |
0.9 mL/min |
|
Gradient: |
50% B to 90% over 10 min, re-equilibrate back to 50% B |
|
Column temp.: |
50 °C |
|
Injection volume: |
2 μL |
|
Run time: |
12 min |
|
Wavelength: |
220 nm |
|
MS scan range: |
200 to 1500 m/z |
|
Cone: |
30 V |
|
Capillary: |
3 kV |
|
Data Management: |
Empower 2 Software |

The structures for polymer additives screened in this method are shown in Figure 1. They cover different classes of additives, such as plasticizers, antioxidants, and UV-absorbers.

Comparing the separation of the standards by each analytical technique, as shown in Figure 2, UPLC and UPC2 were applicable to all 14 compounds chosen. The elution order was different for both methods due to orthogonal selectivity. The ACQUITY UPC2 System provided a shorter run time compared to the ACQUITY UPLC System. It was observed that the thermal instability of some analytes, such as Irganox 1010 and Irganox 245, prevented successful chromatographic separation by GC-MS. Late eluters from Irgafos 168 to Uvitex OB produced wide peaks in GC-MS, possibly due to secondary interactions with the stationary phase or on-column degradation. The compounds selected for this screening were more compatible with liquid chromatography or convergence chromatography than with gas chromatography analysis.

Water extracts analyzed by the ACQUITY UPLC and ACQUITY UPC2 systems did not have any peaks present (data not shown). This was expected, since water is the most common solvent present in the environment. Manufacturers avoid formulating their products to be susceptible to water solubility.
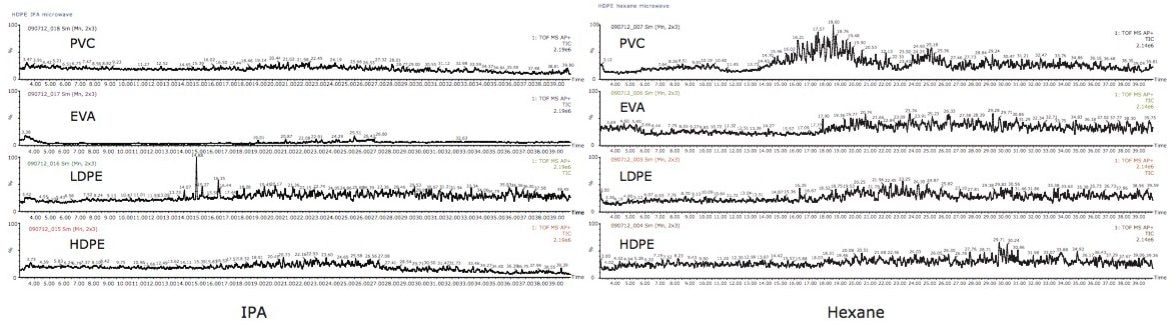
In the other two extracts, hexane and IPA, LDPE had the most extractables present, as seen in Figure 3. IPA extracts analyzed by UPLC (data not shown) produced less intense peaks than UPC2. Prior to UPLC analysis, the hexane extracts were reduced to dryness, re-dissolved in solvent, and analyzed by UPLC (data not shown). Both the ACQUITY UPLC and ACQUITY UPC2 systems showed the same set of extractable compounds present in the samples.

Noisy baselines were observed with the GC-MS analysis. When utilizing this technique, extracted ion chromatograms of known polymers had to be performed, thus making it difficult to screen for unknown extractables in packaging products, as shown in Figure 4. A sample pre-concentration step could have improved the intensity of the detected peaks.
Three known polymer additives were identified in LDPE samples by ACQUITY UPC2, including Irganox 1010, Irganox 1076, and Irgafos 168, as shown in Figure 5. These are commonly used antioxidants that improve the stability of polymers. The identity of each extractable was confirmed by injection of authentic standards, comparison of the retention time, and MS data. An example for Irganox 1076 is shown in Figures 6 and 7. Each of these additives was detected in either hexane or isopropanol extracts of LDPE.
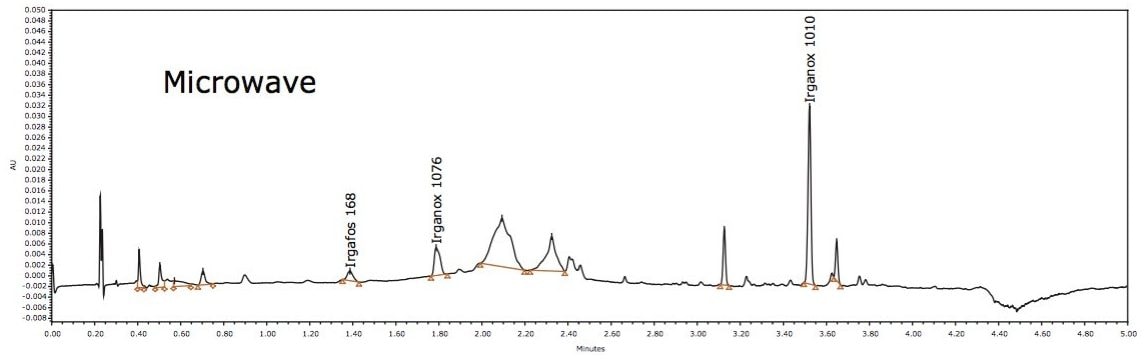

In GC-MS analysis, the presence of Irgafos 168 and Irganox 1076 was also confirmed using standard retention time and mass spectra.
In this application, a single technique was found to be compatible for all extracts of different packaging material. This capability allowed for a streamlined, simplified sample preparation workflow with better asset utilization, since all of the solvent extracts can be directly injected onto the ACQUITY UPC2 System. Using other separation techniques, such as LC and GC, some extracts are not compatible requiring additional processing steps prior to analysis.
UPC2 offered better information for non-volatile and thermally labile compounds than GC due to lower analysis temperatures. The UPC2 analysis provided a two-fold improvement in run time compared to UPLC, and an eight-fold improvement in run time compared to GC.
The ease-of-use coupled with the MS detector provided quick polymer identification for known entities in the sample extracts.
720004490, November 2012