
This work demonstrates how the enhanced sensitivity of the Xevo TQD with fast positive and negative switching, along with tools that facilitate instrument setup and method development, allows for faster analysis of genotoxic impurities.
Allows for faster analysis of genotoxic impurities.
Alkyl sulfonic acids, particularly methanesulfonic acid, benzenesulfonic acid, and p-toluenesulfonic acid, are a common class of alkylating agents used in the pharmaceutical industry as alkylating reagents, catalysts, and in purification steps in the chemical synthesis of an API. In addition, these sulfonic acids are often used as the final salt form of the drug due to improved chemical properties or bioavailability.
The presence of any residual alcohols from synthetic reaction or recrystalization steps may result in the formation of alkyl esters of the sulfonic acids. Many of these mesylate, besylate, or tosylate esters are known to be genotoxic while others are potentially genotoxic, requiring monitoring in the drug substance and drug product.
Typical methods utilized in the past for the analysis of these akyl sulfonate esters have been based on GC-MS or HPLC-UV/MS, with derivitization typically using run times in the order of 20 to 30 minutes We have previously demonstrated how good results can also be achieved using UPLC-MS with run times of less than 5 minutes.
In this application note, we show how the latest advances in instrumentation provide us greater power and ease the analysis of these genotoxic impurities. We demonstrate how the enhanced sensitivity of the Xevo TQD with positive and negative switching allows for faster analysis of these impurities. Additionally, the ability to reduce matrix interference using RADAR helps speed up the method development process.
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC HSS T3 Column, 2.1 x 50 mm, 1.8 μm |
|
Column temp.: |
45 °C |
|
Injection vol.: |
15 μL (20 μL loop partial loop mode) |
|
Mobile phase A: |
98 H20 2% MeOH 0.1% Formic acid |
|
Mobile phase B: |
98 MeoH 2% H2O 0.1% Formic acid |
|
Time (min) |
Flow Rate |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.6 |
95 |
5 |
|
|
2.5 |
0.6 |
2 |
98 |
6 |
|
3 |
0.6 |
2 |
98 |
6 |
|
4 |
0.6 |
95 |
5 |
1 |
|
4.5 |
0.6 |
95 |
5 |
1 |
|
Capillary voltage: |
0.8 kV |
|
Function type: |
MRM of 3 channels |
|
Chan reaction |
Cone voltage |
Col. Energy |
Compound |
|---|---|---|---|
|
95.00 > 79.90 |
40 |
15 |
methanesulfonic acid |
|
157.00 > 79.90 |
45 |
24 |
benzenesulfonic acid |
|
171.00 > 79.90 |
48 |
26 |
toluenesulfonic acid |
|
Capillary voltage: |
0.5 kV |
|
Chan reaction |
Cone voltage |
Col. Energy |
Compound |
|---|---|---|---|
|
173.10 > 77.00 |
25 |
16 |
methylbenzene sulfonate |
|
187.00 > 77.00 |
25 |
22 |
ethyl benzenesulphonate |
|
187.00 > 155.00 |
30 |
10 |
methyl p-toluenesulfonate |
|
201.00 > 173.00 |
25 |
10 |
ethyl p-toluenesulfonate |
|
229.10 > 91.00 |
40 |
20 |
2S glycidyl tosylate |
Standard solutions of a number of known genotoxic impurities were prepared at 1 mg/mL in acetonitrile and then diluted to concentrations from 0.1 to 500 ng/mL in 5% acetonitrile. In addition, a solution prepared by crushing and dissolving a 10 mg amplodipine besylate tablet, spiked with 1.5 µg genotoxic impurities, was also prepared.
The individual standard solutions were used to help tune the MS using IntelliStart.
Even under the best chromatographic performance coelution can occur. Implementing an approach whereby qualitative MS scan data obtained from the matrix is simultaneously acquired with quantitative multiple reaction monitoring (MRM) MS data can aid in the monitoring of potential interfering compounds, ensuring assay robustness and reproducibility.
In RADAR mode, MRM data can be collected in parallel to the collection of spectral MS data, in both positive and negative ion modes. This can be done with little or no impact on the quality of the MRM data. As a result, you can accurately quantify target compounds while at the same time track other sample matrix components, arming you with a greater depth of knowledge about your sample.
It is important to recognize that RADAR is only possible because of the Xevo TQD’s ability to rapidly alternate between MS, MS/MS, positive, and negative ion modes without compromising performance.
RADAR was used to assist in the method development for the LC-MS/MS method for the analysis of the impurities in the spiked tablet solution. The final method developed was used to demonstrate the linearity and sensitivity the system for the genotoxic impurities.
Figure 1 shows how RADAR was used to aid in the method development of the LC-MS/MS method for the analysis of the impurities in the tablet formulation solution.
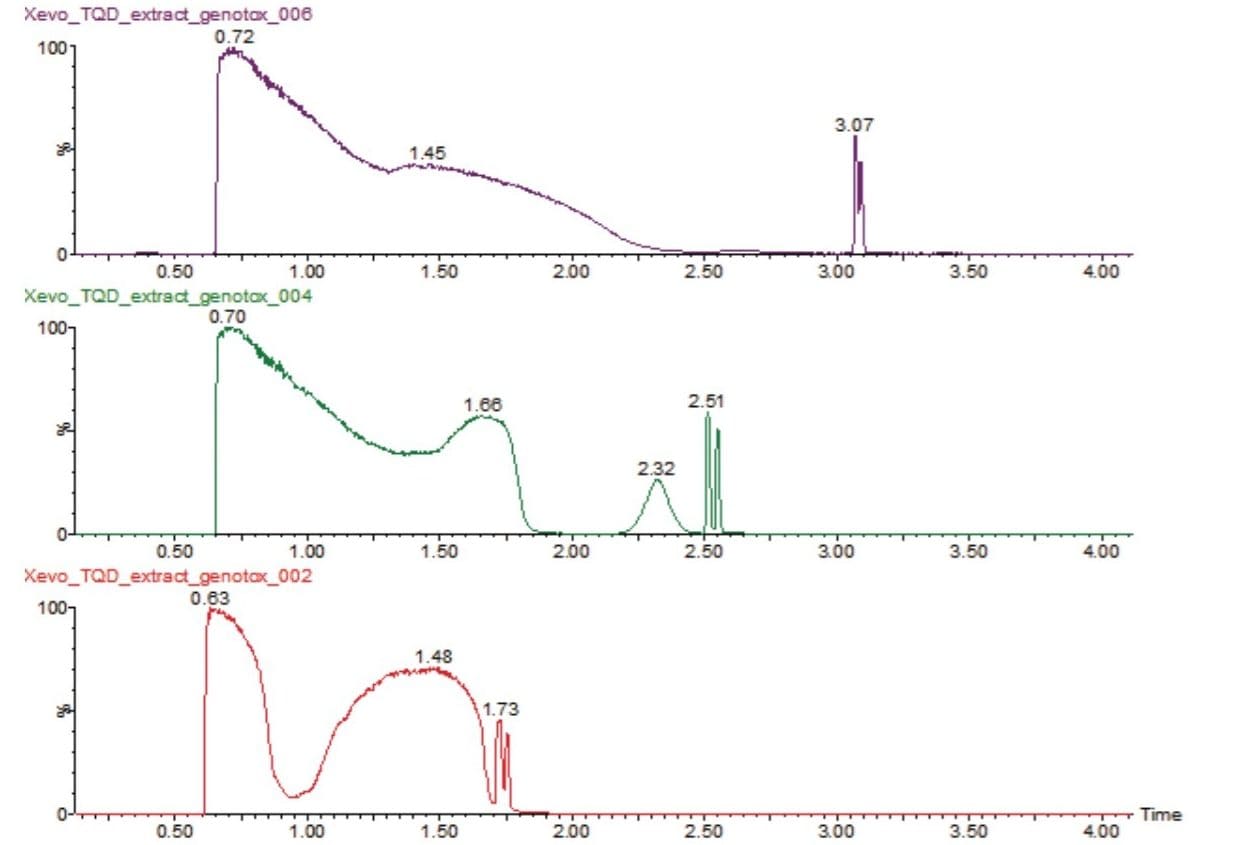
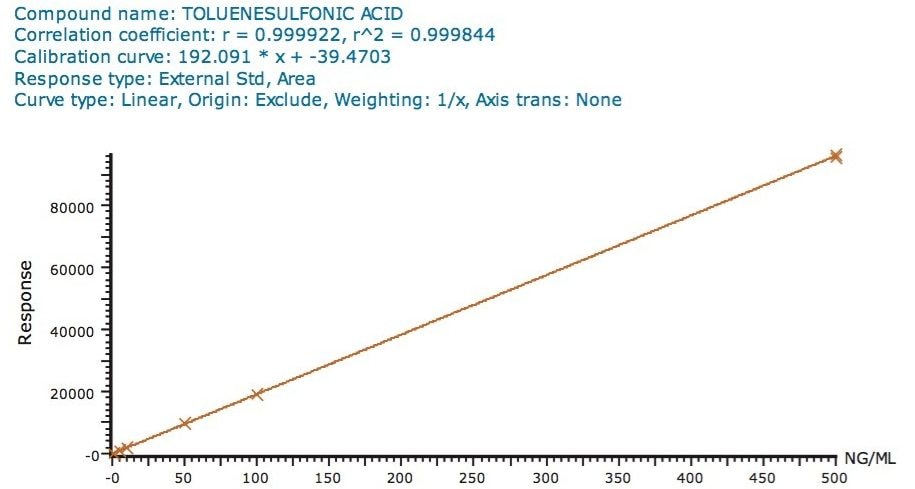
Figure 2 shows the linearity plot obtained for toluene sulfonic acid in the range of 0.1 to 500 ng/mL.
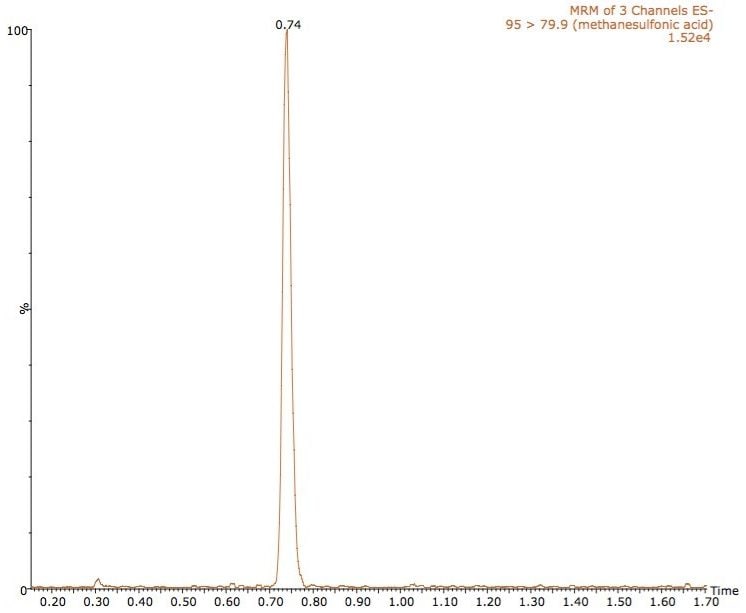
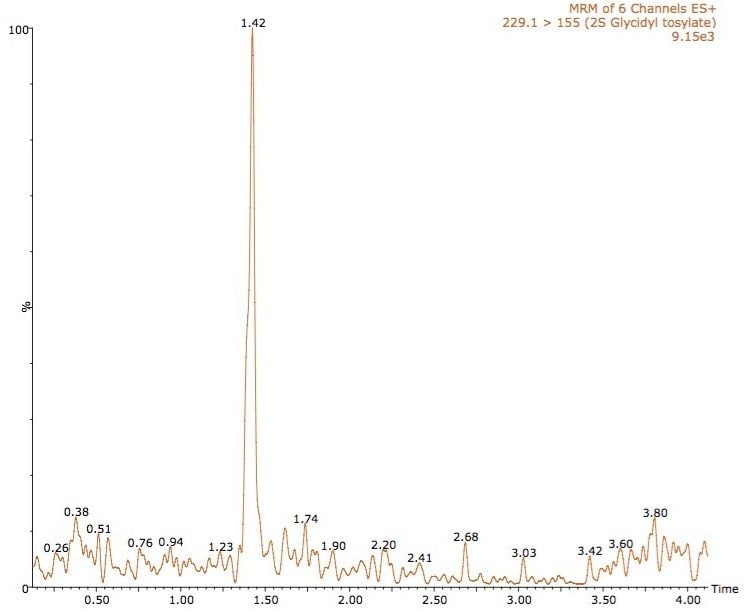
An example chromatogram of methane sulfonic acid at 0.1 ng/mL is shown in Figure 3, and glycidyl tosylate in Figure 4.
The introduction of the tandem quadrupole detector Xevo TQD has added an extra tool for the analysis of genotoxic impurities when paired with UPLC. It offers sensitive and rapid methods that can be developed quickly and easily through the use of multiple features such as IntelliStart and RADAR, positive/negative switching, and the generation of “all of the data all of the time.” This allows for significant improvements in laboratory productivity at a time when scientific and regulatory demands are increasing.
720004012, June 2011