This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to successfully characterize impurities in synthesized fine chemicals by exact mass MS/MS using FastDDA on the Xevo G2 QTof.
FastDDA automatically produces MS/MS product ion spectra that can be used to successfully elucidate the structure of trace level impurities.
Analytical laboratories studying the products of organic synthesis have to consider many things from confirmation of the final product to identification of impurities. Impurity identification, whether expected or not, is an essential part of the manufacture of fine chemicals, as any impurities could adversely affect the properties of the final product. This applies equally to the raw starting materials and the final synthesized product.
Obtaining MS/MS product ion spectra is one useful way of elucidating the structure of impurities, while exact mass provides additional confidence to structural assignments. However, the manual setup of each product ion experiment can be time consuming and prone to human error, so a Data Directed Analysis (DDA) is beneficial with regard to laboratory resources. DDA automatically selects ions for MS/MS acquisitions in real time, as components elute from the column. Recent improvements in the spectral acquisition rate (30 Hz) now enable DDA to be compatible with UPLC peaks.
Octahydroacridine (>97% purity) was used here for illustration purposes. Base peak intensity (BPI) chromatogram and extracted ion chromatograms of some of the impurities found automatically by FastDDA are shown in Figure 1. Octahydroacridine is interesting as it plays an important role in the preparation of alkaloids, dyes, drugs, and other biologically active compounds.
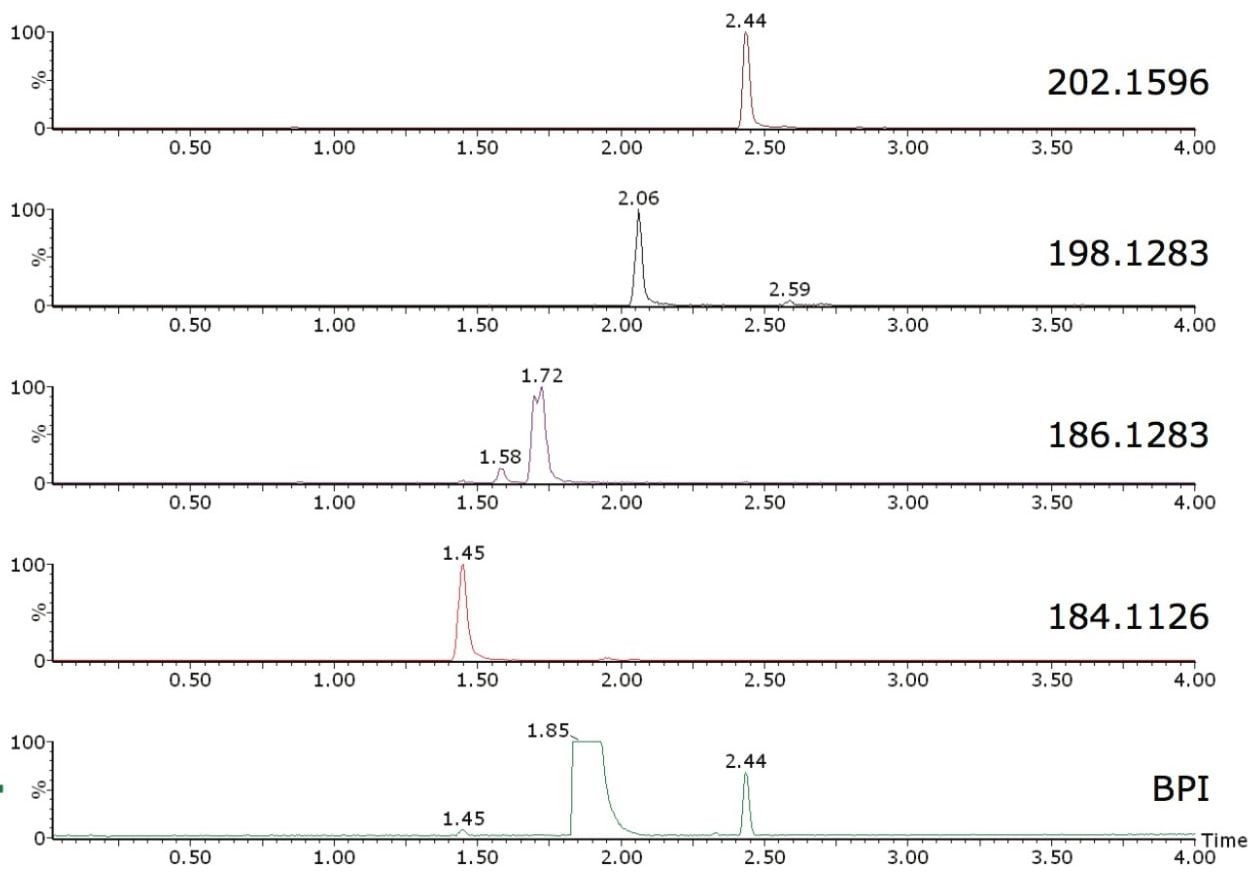
Waters Xevo G2 QTof, coupled with the ACQUITY UPLC System and operated in FastDDA acquisition mode, rapidly and automatically generated MS/MS product ion spectra of all the impurities, enabling structural elucidation to be performed with MassFragment Software.
FastDDA intelligently selects ions for MS/MS acquisitions in real time, as components elute from the chromatographic system. Embedded algorithms rapidly interrogate MS survey spectra and co-eluting precursor ions are selected for MS/MS analysis based on threshold intensity and pre-defined exact mass include/exclude lists.
In this example, octahydroacridine (m/z 188.1439) was excluded from the FastDDA setup as the expected compound. This setup allowed seven discrete impurities to be characterized with the automatic generation of high resolution MS/MS product ion spectra, four of which are shown in Figure 2.

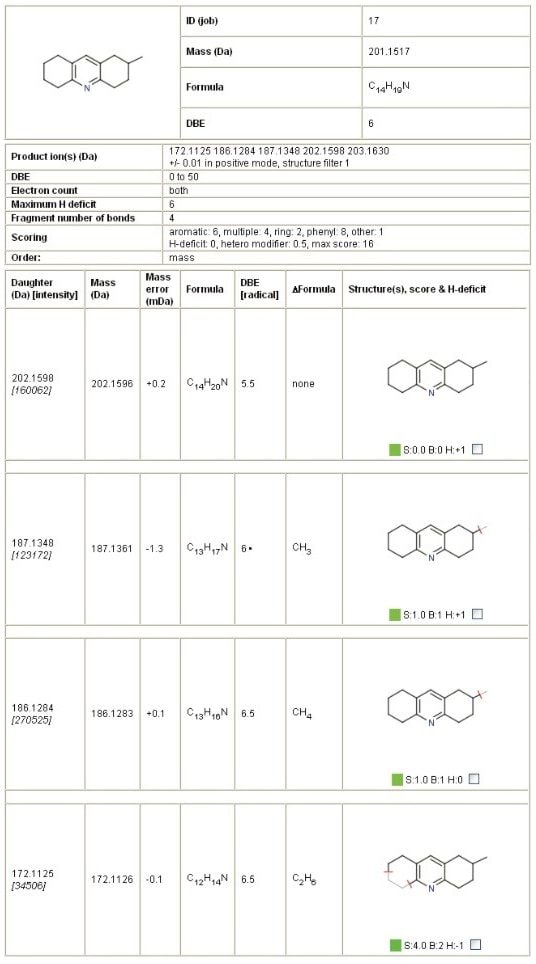
MassFragment Software, the automated structural elucidation tool which uses a systematic bond cleavage and ranking algorithm, was employed to rationalize and identify fragment ion structures from potential impurities. By submitting a postulated precursor structure and a MS/MS product ion spectrum, the MassFragment Software tool generates a report of possible fragmentation with exact mass confirmation. An extract from the MassFragment results summary for the impurity, m/z 202.1596, is shown in Figure 3.
The synthesized product and trace-level impurities were successfully characterized using the:
The rapid, automated nature of this solution minimizes the need for manual intervention, which reduces the drain on laboratory resources and maximizes the information obtained from a single analytical experiment.
720003838, January 2011