For research use only. Not for use in diagnostic procedures.
This application note describes the efficient coupling of an Open Architecture UPLC System configuration with online radioactivity detection (radio-UPLC) and demonstrates the improvements obtained relative to traditional radio-HPLC for identification of metabolites.
The combination of Open Architecture UPLC with online radioactivity detection equipped with newly-designed admix or solid cells can significantly improve the separation and data quality for the identification of radio labelled metabolites.
The study of the metabolism of a compound plays a key role in drug discovery and development and contributes to the optimization of drug candidates and to the support of toxicity studies. The identification of metabolites in complex biological matrices is challenging. Metabolites are structurally-related entities and structure differences can be very minor. Therefore, we are faced with the challenge of quantifying very low levels of individual metabolites that are difficult to chromatographically separate due to their structural similarity.
Ultra performance liquid chromatography (UPLC) systems and columns have become one of the most efficient solutions to improve chromatographic performance in many areas of small molecule analysis, including metabolite profiling and identification. Since baseline separation is required for accurate metabolite quantification with radiochemical detection – the method of choice in this field – its successful combination with UPLC will result in notable improvements in data quality. The major challenge for a successful radio-UPLC implementation is that the narrow peak widths obtained in UPLC separations appear to be intrinsically incompatible with the relatively high counting times needed to achieve appropriate sensitivity in radiochemical detection.
This application note describes the efficient coupling of an Open Architecture UPLC System configuration with online radioactivity detection (radio-UPLC) and demonstrates the improvements obtained relative to traditional radio-HPLC for identification of metabolites.
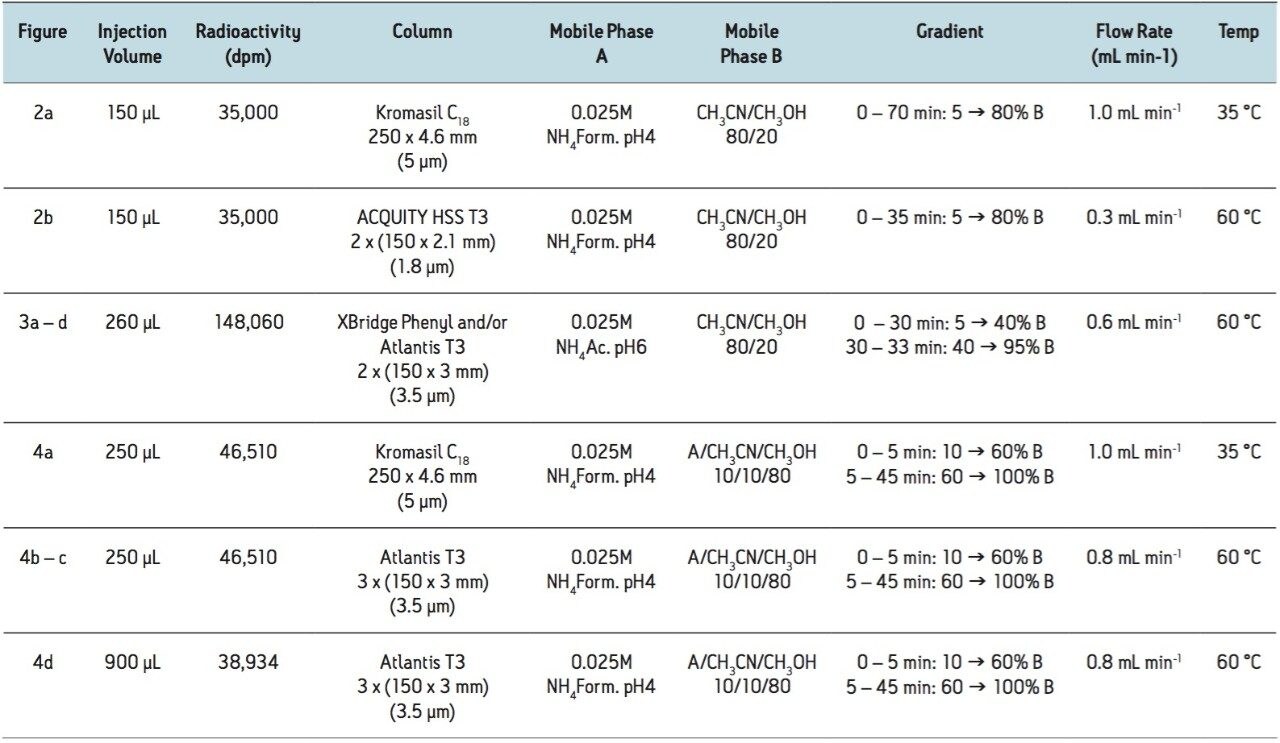
Figure 1 shows the Waters Open Architecture UPLC System. In this study, this system was coupled to a Berthold Radioactivity Monitor LB509 equipped with a Z-200-6M (200 μL) admix cell. The scintillation cocktail was added with a Berthold scintillator pump via a home-made variable scintillation flow setup as earlier described.1 The implementation of the variable scintillation flow via a simple modification to the classical on-line radiochemical detection configuration results in improved sensitivity, while significantly reducing scintillation liquid consumption and radioactive waste production.

The radio-UPLC analyses shown in Figures 4b to 4d were obtained with a YG-75-S6M (75 μL) or YG-150-S6M (150 μL) solid cell.
Standard radio-HPLC profiles were generated for comparison with a Waters Alliance 2695 HPLC System coupled to a Berthold Radioactivity Monitor LB509 equipped with a Z-1000 (1000 μL) admix cell. The eluates were mixed with scintillation cocktail delivered by a Berthold LB 5035-3 pump at a flow rate of 4 mL/min.
The detector outputs were entered into Waters Empower 2.0 Software via an analogue output. The possibility to import Berthold data digitally into Empower would further improve the signal-to-noise ratio of the radiochromatograms shown in this application note.
The chromatograms obtained by injection of 150 μL of a 120 min incubation of a 3H-labelled drug in rabbit hepatocytes suspension culture on radio-HPLC and radio-UPLC are illustrated in Figure 2. The separation of the individual metabolites in this complex metabolite mixture was significantly improved using radio-UPLC, and as an additional benefit the time of analysis was reduced by half. This can be clearly noted for the metabolites at 20.0, 29.5, 30.5 and 40.5 min that are almost completely coeluting in the original radio-HPLC trace, as shown in Figure 2a, while in comparison they were mostly baseline separated in the radio-UPLC chromatogram, shown in Figure 2b, allowing for better quantification with online radioactivity detection. The gain in resolution is clearly demonstrated in the peak widths of the metabolite eluting at 46 min and the parent drug eluting around 65 min, where there is a reduction from ± 70 sec at the baseline (FWHM = 30 and 20 sec, respectively) in radio-HPLC to ± 14 sec (FWHM = 7 and 8 sec, respectively) in radio-UPLC.
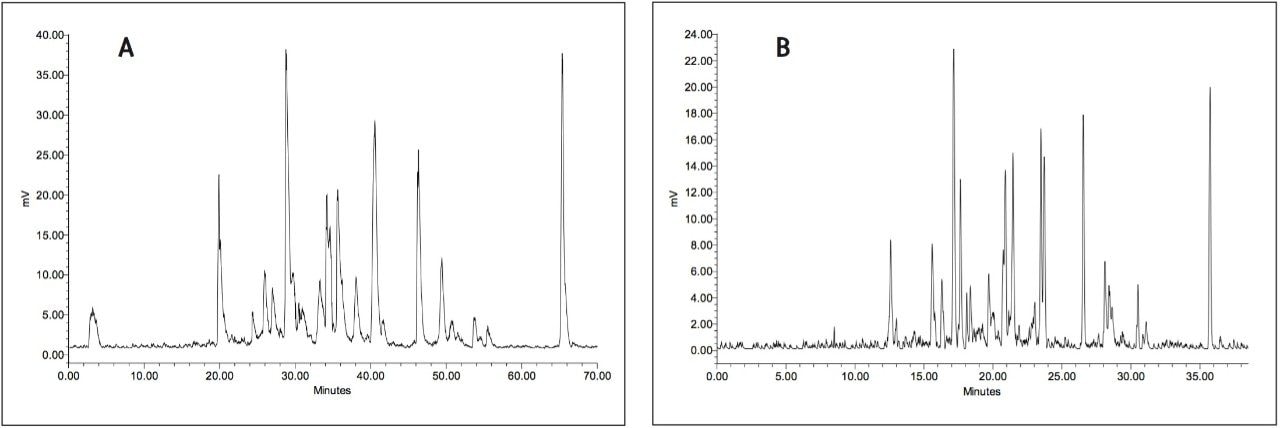
In this example, the RAD detector was equipped with an admix cell of reduced volume (200 μL) and internal diameter1; a further reduction of the cell size would result in even better resolution. This would, however, only be useful for applications providing samples with high radioactive concentrations2 since a reduction in cell size also proportionally reduces sensitivity. The 200 μL cell was chosen (instead of 1000 μL used in radio-HPLC) for this application as a compromise between sensitivity and resolution taking into account the low radioactive concentrations generally encountered in samples of metabolism studies.
For the same reason, two 150-mm columns were coupled for this analysis, allowing a two-fold larger injection volume, while also increasing separation power.
The coupling of columns also allows for experimentation with different column chemistries. This is illustrated in Figure 3 where the analysis of a 24 h rabbit urine sample on an XBridge Phenyl Column coupled with an Atlantis T3 Column (shown in Figure 3b) and vice versa (shown in Figure 3c) is compared to the analysis of the same sample on two columns of the same chemistry. The radiochromatograms obtained with two different column chemistries show an intermediate profile compared to the ones obtained with only XBridge Phenyl (shown in Figure 3a) or Atlantis T3 (shown in Figure 3d) columns. The first column seems to have a slightly dominant effect on the separation as can be derived from the separation of the three metabolites eluting around 24.5 min and a minor shift in retention in agreement with the earlier elution on the XBridge Phenyl Column and longer retention on the Atlantis T3 Column.
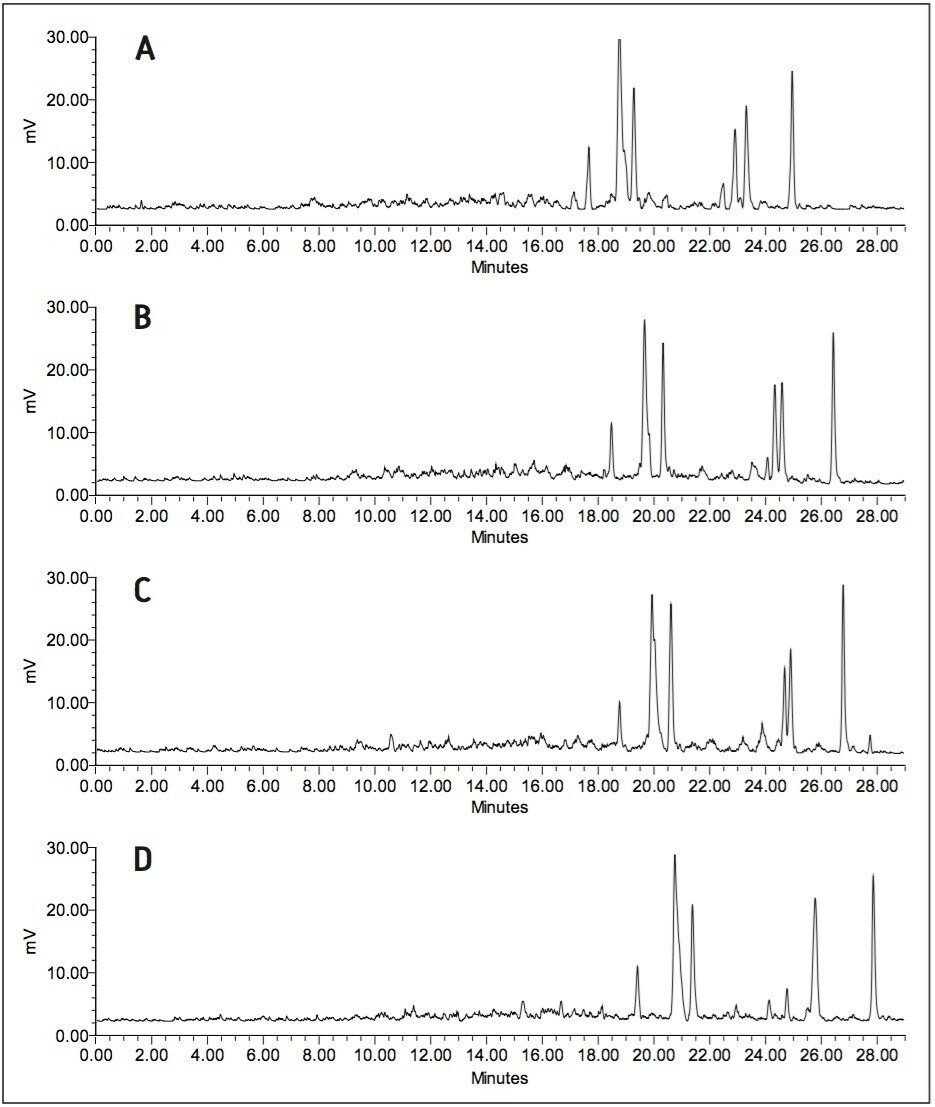
While the XBridge Phenyl Column Chemistry provides the best separation for this sample as expected based on the poly-aromatic structure of the parent drug, the coupling of two columns with different selectivities can be beneficial for metabolism scientists seeking a more generic approach or for samples where different pairs or groups of metabolites can only be chromatographically separated with different column chemistries.
For the experiment illustrated in Figure 4, 3-mm I.D. columns packed with 3.5-μm particles were used instead of the 2.1-mm I.D. sub-2-μm particles traditionally used for UPLC. The main reason for their selection is that larger injection volumes are tolerated by larger I.D. columns. The loss of resolution and sensitivity is acceptable for radioactive profiling since peak width is affected by the use of relatively large admix cells that are necessary to ensure adequate residence time in the detector, which is necessary to provide the required sensitivity for the low radioactive concentrations encountered in metabolism samples. (The use of 1.7-μm particle packed 3-mm I.D. columns was not explored in this work.)
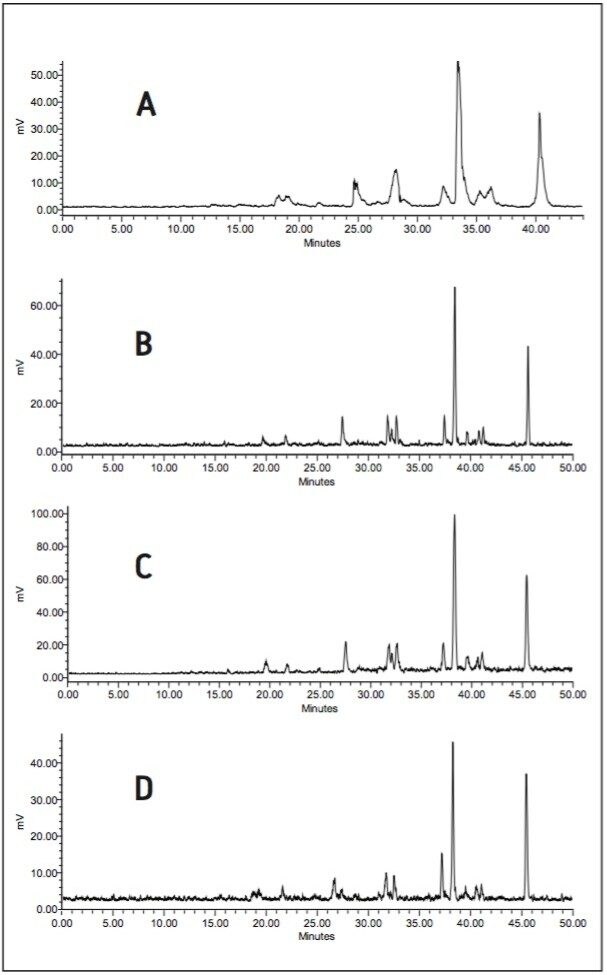
The application of large volume injections is illustrated in Figure 4d where 900 μL of a 4 h bile sample was injected on three 3-mm I.D. columns. The ACQUITY UPLC System provided sufficient backpressure to use the three coupled columns at an optimal flow rate. The open architecture configuration makes the injection of large injection volumes possible. As a result, this bile sample of relatively low radioactive concentration could be injected on-column to deliver a good quality radiochromatogram with online radioactivity detection. Compared to the standard radio-HPLC chromatogram, shown in Figure 4a, the separation and resolution were significantly improved in an almost identical analysistime, Figures 4b and 4c.
Instead of the 200 μL admix cell, a solid cell was used for the latter two radiochromatograms. From the peak width and shape obtained, it can be derived that the advancements made for the admix cells are also true for the newly-designed Berthold solid cells. A YG75-S6M solid cell (75 μL) was used for the analysis in Figure 4b, while a YG150-S6M solid cell (150 μL) was used for the analysis in Figure 4c. The YG75-S6M cell shows good resolution with sensitivity for 14C labelled compounds comparable to a 200 μL admix cell. The YG150-S6M cell is two-fold larger resulting in a two-fold longer residence time in the radiodetector. The increase in peak intensities was, however, only ± 30% relative to the smaller YG75-S6M cell. This can be explained by the increasing background noise, but more importantly by the loss in resolution caused by diffusion in the larger cell.
This application note demonstrates how the combination of Open Architecture UPLC with online radioactivity detection equipped with newly-designed admix or solid cells can significantly improve the separation and data quality for the identification of radio labelled metabolites. When used with coupled, large I.D. columns, the Open Architecture UPLC System enables the injection of large volumes of metabolite samples with low radioactive concentrations. By coupling columns, different column chemistries can be combined, which provides additional selectivity for the separation.
720003782, October 2010