
This application note demonstrates to develop a rapid and sensitive quantitative method from sample preparation to data analysis for melamine and cyanuric acid.
Numerous incidences of kidney stones and renal failure in infants have been reported in China since July 2008, believed to be associated with the ingestion of infant formula intentionally contaminated with melamine. Allegedly, nitrogen-rich melamine was added to raw milk to boost the apparent protein content which is assessed through determination of the nitrogen content by the Kjeldahl or Dumas method.
Melamine has many industrial uses, including the production of laminates, adhesives and melamine resins, some of which may contact foods, leaving trace level of detectable residues. Additionally, melamine has been reported to be a metabolite of the pesticide cyromazine. Taking these widespread sources into account, the oral uptake of melamine has been estimated at 0.007 mg/kg body weight/day.1
A tolerable daily intake (TDI) has been established by the US FDA at 0.63mg/kg body weight per day2 in food other than infant formula and the TDI quoted by the European Food Safety Authority (EFSA) is in broad agreement at 0.5 mg/kg body weight.3 More recently US FDA has applied an additional 10-fold safety factor for infant formula.7 This results in a TDI of 0.063 mg melamine/kg body weight per day for infants. Infants may be more sensitive than adults to exposures because infant formula is the sole source of nutrition, and renal function may be more immature compared to adults.
Maximum permitted concentrations for melamine in adult foods are typically around 2.5 μg/kg (EU, US, and Hong Kong); however, Hong Kong has set a tolerance at 1 μg/kg in infant foods. US FDA has stated that no tolerance can be set in infant formula4 and Taiwan has declared that melamine should not be detected in any food using the most sensitive instrumentation.
Cyanuric acid is a structural analogue of melamine and may be found as an impurity of melamine. US FDA permits its use as a non-protein nitrogen additive in animal feed. Cyanuric acid may also be found in swimming pool water as the dissociation product of dichloroisocyanurates used for sanitization. Cyanuric acid has low acute toxicity in mammals. The oral LD50 of cyanuric acid is 7,700 mg/kg body weight for rats (OECD 1999). Several sub-chronical oral toxicity studies have shown that it can damage the renal tissue, and the no-observed-adverse-effect-level (NOAEL) is 150 mg/kg/day.
During the 2007 pet food recall in North America, melamine-tainted pet foods caused significant renal damage in dogs and cats. While individually melamine and cyanuric acid post low acute toxicity, a mixture of these two compounds forms an insoluble precipitate in renal tubules leading to progressive tubular blockage and degeneration.5
There is, therefore, a need to develop methods of detection for both melamine and cyanuric acid capable of quantifying both compounds in infant formula milk products from ppm levels down to very low ppb levels. US FDA issued an interim method for determining residual melamine and cyanuric acid in foods using LC-MS/MS.6 This method was validated for the determination of melamine and cyanuric acid in both pork and fish tissue, but has only been evaluated for performance in infant formula. In this method, melamine and cyanuric acid are extracted from infant formula with a 50/50 acetonitrile-water solution. After centrifugation, two aliquots of each extract were individually cleaned up using two different mixed-mode solid-phase extraction (SPE) procedures. Oasis MCX was used to selectively extract melamine from the sample and Oasis MAX was used to selectively extract cyanuric acid. HILIC is the FDA suggested LC method. Two HILIC methods were developed for this work to satisfy the needs of both HPLC and UPLC users. Columns employed in these methods were the Atlantis HILIC Silica and the ACQUITY UPLC BEH HILIC. Both methods were developed using the ACQUITY UPLC with TQD. This application note outlines the only complete single vendor solution for the quantitative analysis of melamine and cyanuric acid in infant formula.
All standard and sample solutions are prepared per the FDA interim method. Stock melamine and cyanuric acid standards (TCI America, USA) were prepared separately at 10 μg/mL in 50:50 acetonitrile:water. The stock standard solutions were used to prepare calibration standards and spiked samples.
Isotopically-labelled standards of 13C3 15N3 melamine and 13C315N3 cyanuric acid were purchased from Cambridge Isotope laboratories, Inc. Stock solutions of the 13C3 15N3 melamine and 13C3 15N3 cyanuric acid were made at 1 μg/mL and 10 μg/mL, respectively.
For melamine, calibration standard solutions were prepared at 0, 1, 10, 50, 250, and 1000 ng/mL of melamine in 2% diethylamine/acetonitrile. Each calibration standard contained isotopically-labelled melamine at 10 ng/mL.
For cyanuric acid, calibration standard solutions were prepared at 0, 10, 50, 200, 500, and 1000 ng/mL of cyanuric acid in 4% formic acid in acetonitrile. Each calibration standard contained isotopically-labelled cyanuric acid at 100 ng/mL.
The calibration standards and sample extracts for melamine and cyanuric acid were prepared and analyzed separately.
Dry and liquid infant formulas were obtained from a local supermarket for use in recovery experiments.
|
LC System: |
Waters ACQUITY UPLC System |
|
Column: |
Atlantis HILIC Silica 2.1 x 150 mm, 3 μm |
|
Part Number: |
186002015 |
|
Injection Volume: |
20 μL |
|
Mobile phase A: |
10 mM Ammonium acetate in 50/50 Acetonitrile/H2O |
|
Mobile phase B: |
10 mM Ammonium acetate in 95/5 Acetonitrile/H2O |
|
Time (min) |
Flow Rate (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.5 |
0 |
100 |
- |
|
2 |
0.5 |
0 |
100 |
6 |
|
3.5 |
0.5 |
60 |
40 |
6 |
|
5 |
0.5 |
60 |
40 |
6 |
|
5.2 |
0.8 |
0 |
100 |
6 |
|
11 |
0.8 |
0 |
100 |
6 |
|
11.1 |
0.5 |
0 |
100 |
6 |
|
8.14 |
0.5 |
0 |
100 |
6 |
|
MS System: |
Waters ACQUITY TQD |
|
Software: |
Waters MassLynx v.4.1 |
|
Ionization Mode: |
ESI Positive (melamine and 13C3 15N3 melamine) ESI Negative (cyanuric acid and 13C3 15N3 cyanuric acid) |
|
Capillary Voltage (kV): |
3 |
|
Source Temp (°C): |
150 |
|
Desolvation Temp (°C): |
400 |
|
Cone Gas Flow (L/Hr): |
50 (Nitrogen) |
|
Desolvation Gas Flow (L/Hr): |
900 (Nitrogen) |
|
Collision Gas: |
Argon at 3 x 10-3 mBar |
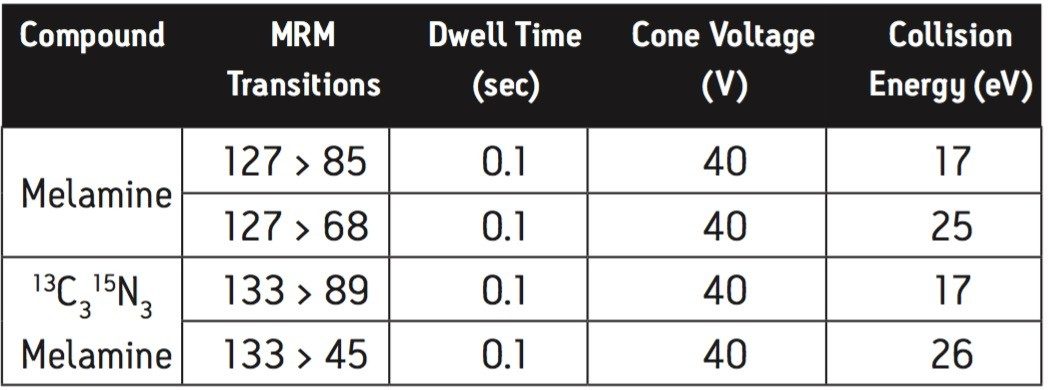
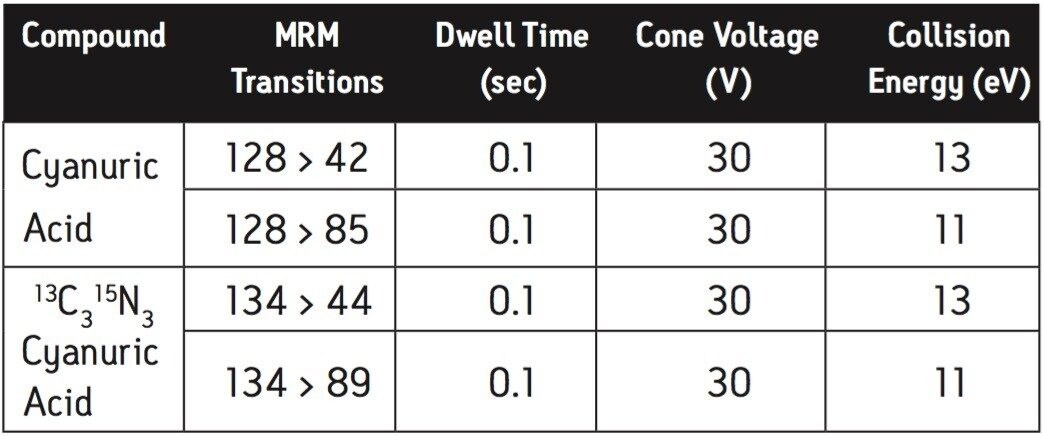
Two multiple reaction monitoring (MRM) transitions were monitored for each compound to meet relevant criteria for identification and confirmation. The details of ESI positive MRM transitions, dwell times, cone voltages, and collision energies for melamine and 13C3 15N3 melamine are shown in Table 1. Cyanuric acid and 13C3 15N3 cyanuric acid were analyzed under ESI negative conditions: the MRM transitions, dwell times, cone voltages, and collision energies are summarized in Table 2.
|
LC System: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH HILIC 2.1 x 100 mm, 1.7 μm |
|
Part Number: |
186003461 |
|
Injection Volume: |
10 μL |
|
Mobile phase A: |
10 mM Ammonium acetate |
|
Mobile phase B: |
10 mM Ammonium acetate in 95/5 Acetonitrile/H2O |
|
Time (min) |
Flow Rate (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.6 |
0 |
100 |
- |
|
0.8 |
0.6 |
0 |
100 |
6 |
|
2.3 |
0.6 |
22 |
78 |
6 |
|
2.8 |
0.6 |
22 |
78 |
6 |
|
2.9 |
0.6 |
0 |
100 |
6 |
|
6.4 |
0.6 |
0 |
100 |
6 |
The MS conditions are the same as those listed in the earlier section for the HPLC analysis.
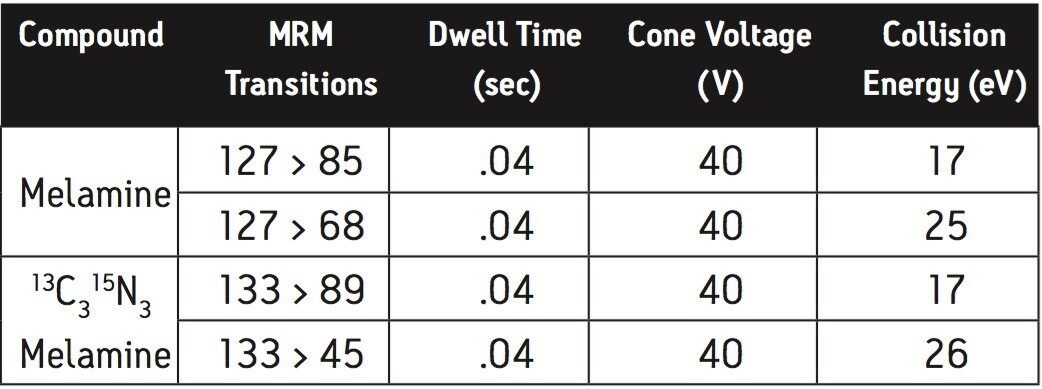
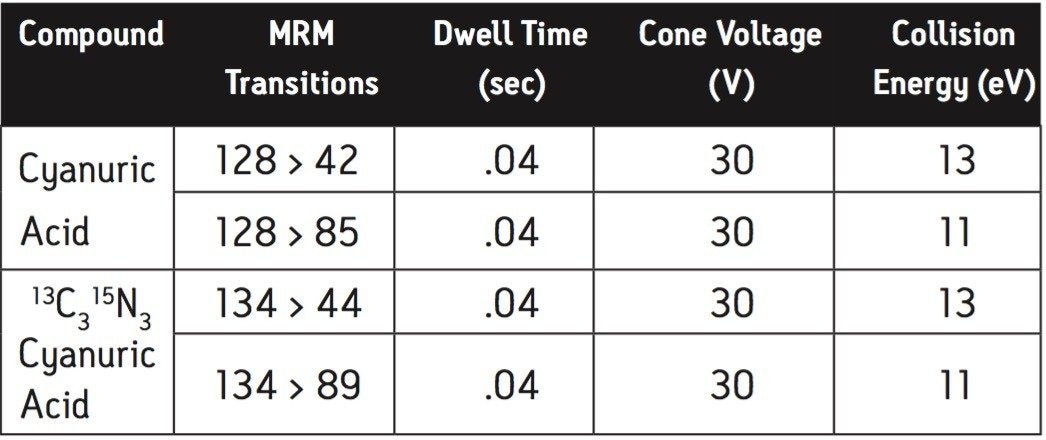
Two multiple reaction monitoring (MRM) transitions were monitored for each compound to meet relevant criteria for identification and confirmation. The details of ESI positive MRM transitions, dwell times, cone voltages, and collision energies for melamine and 13C3 15N3 melamine are shown in Table 3. Cyanuric acid and 13C3 15N3 cyanuric acid were analyzed under ESI negative condition: the MRM transitions, dwell times, cone voltages, and collision energies are summarized in Table 4.
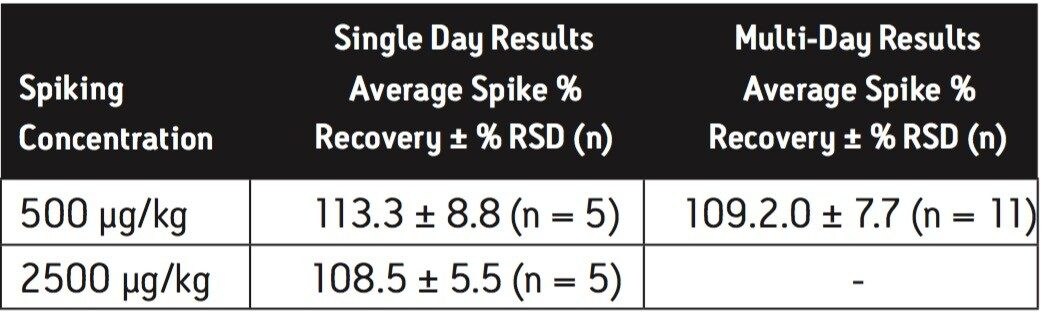
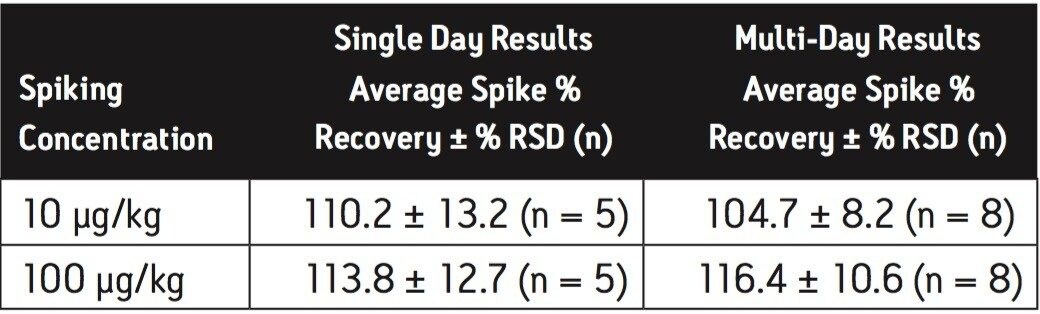
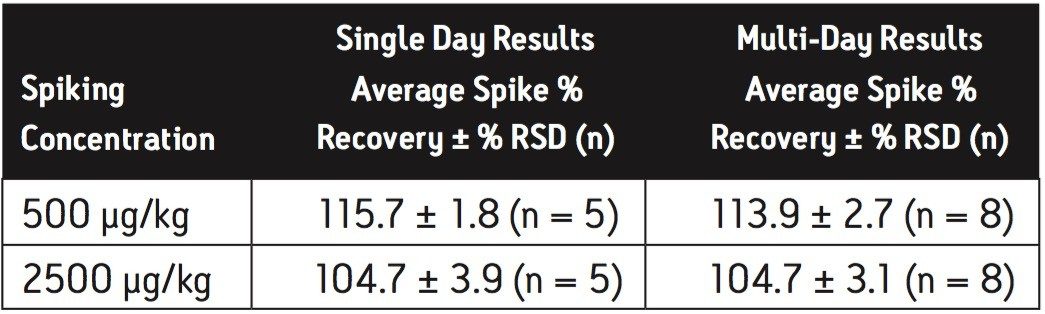
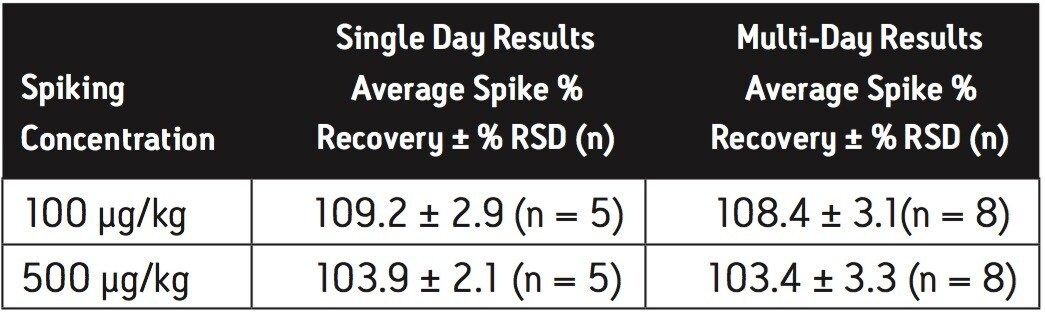
Extraction efficiency for the method was evaluated by fortification of infant formula at the concentrations indicated in the US FDA interim method,6 in replicate at each concentration. Melamine was spiked at 500 μg/kg and 2500 μg/kg in the dry baby formula, and spiked at 10 μg/kg and 100 μg/kg in the liquid baby formula. Cyanuric acid was spiked at 500 μg/kg and 2500 μg/kg in the dry baby formula, and spiked at 100 μg/kg and 500 μg/kg in the liquid baby formula. The experiment was repeated on a second day and the overall data were presented as multi-day results. The high level melamine spiking experiment at 2500 μg/kg was not part of the multi-day study for dry infant formula. Melamine and cyanuric acid extracts were analyzed separately on both the HILIC Columns and the ACQUITY BEH HILIC Column and Atlantis HILIC Silica Column.
Average melamine spike recoveries in dry baby formula and liquid formula were ranged from 110% to 115% (Table 5) and from 104% to 106% (Table 6), respectively. Average cyanuric acid spike recoveries in dry baby formula and liquid baby formula were ranged from 105% to 116% (Table 7) and from 103% to 118% (Table 8), respectively. Typical chromatograms of melamine and cyanuric acid are shown in Figures 1 and 2.
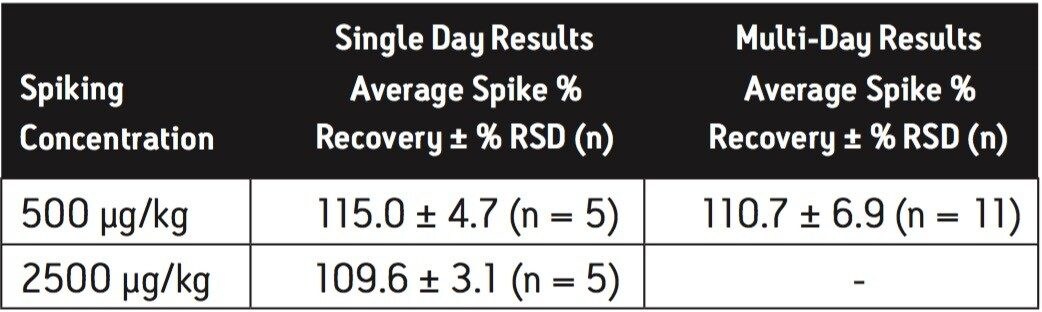
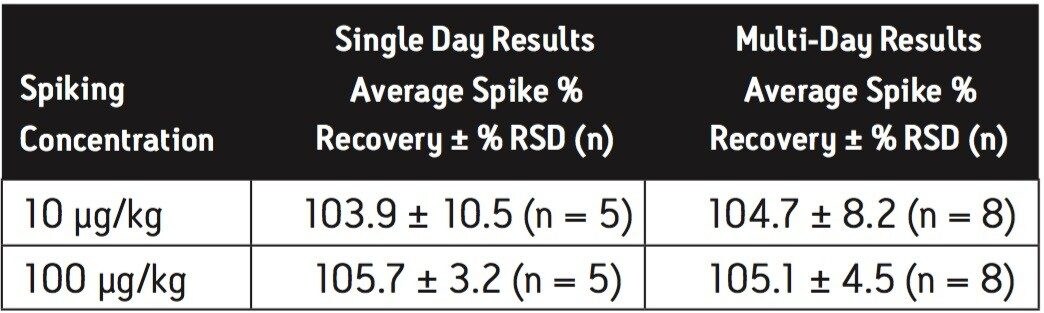
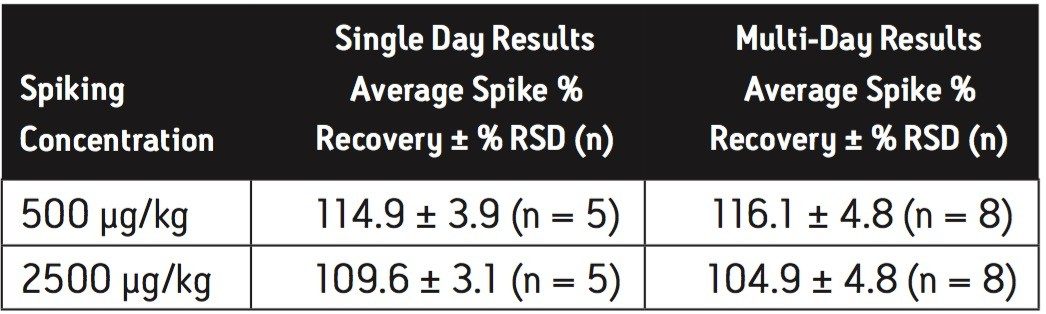
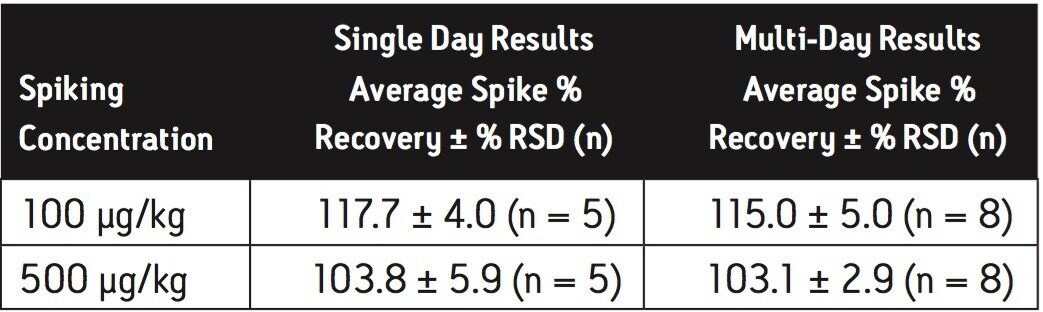
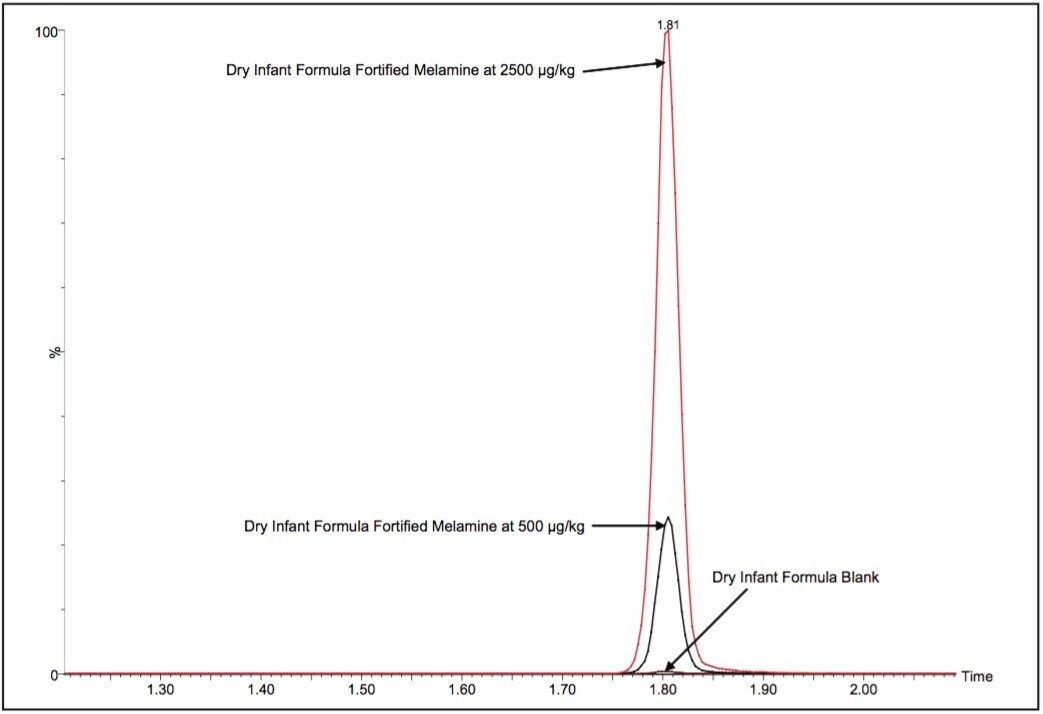
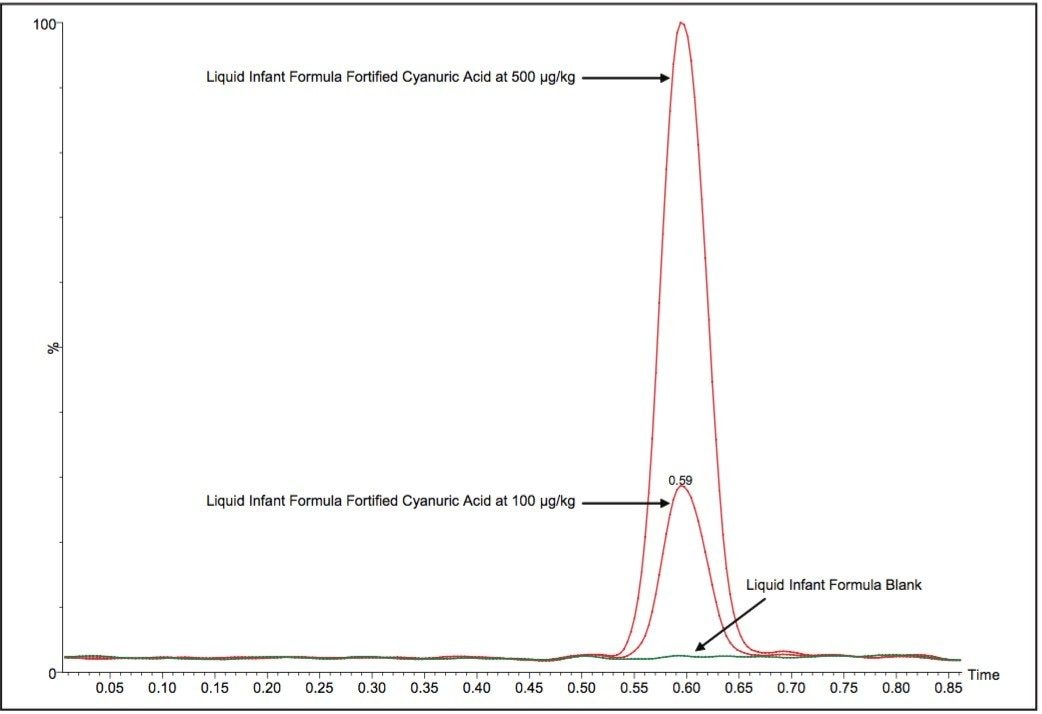
Average melamine spike recoveries in dry baby formula and liquid infant formula were ranged from 109% to 113% (Table 9) and from 105% to 116% (Table 10), respectively. Average cyanuric acid spike recoveries in dry infant formula and liquid infant formula were ranged from 105% to 116% (Table 11) and from 103% to 109% (Table 12), respectively. Typical chromatograms of melamine and cyanuric acid were shown in Figures 3 and 4.
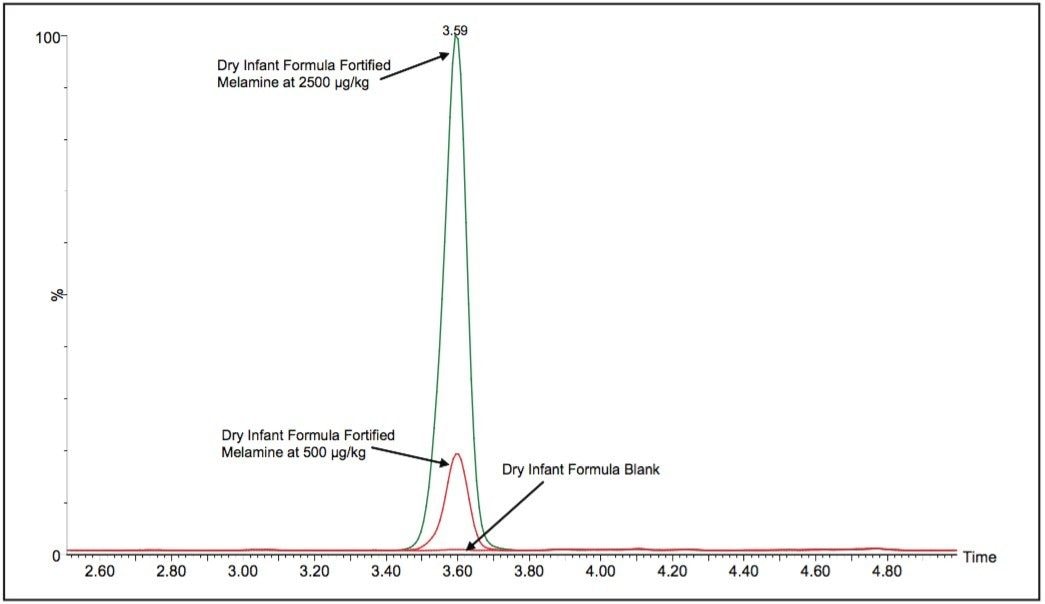
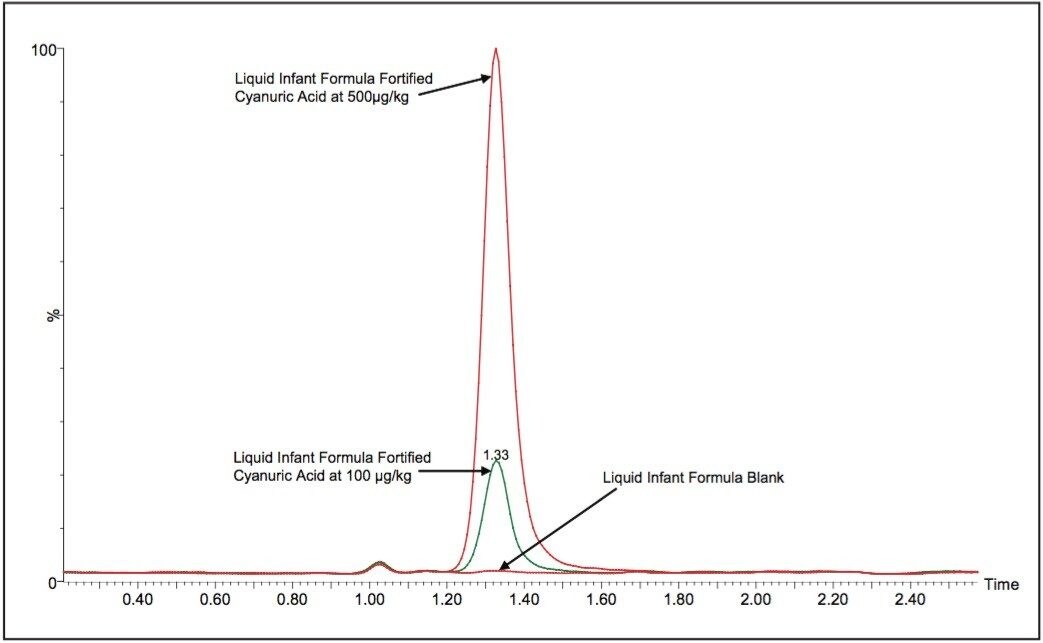
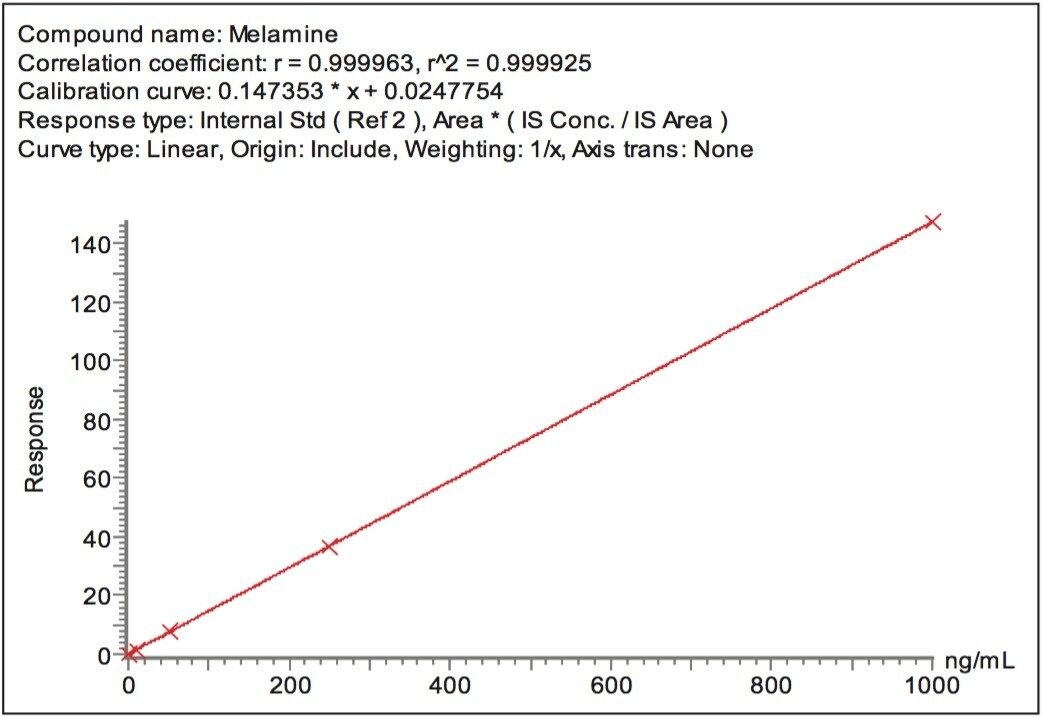
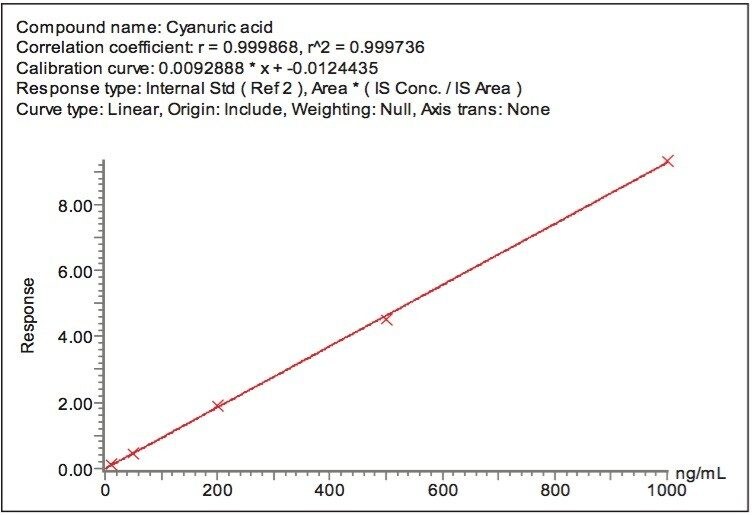
By including stable isotope-labelled internal standards in the extracts, the linearity of the method extended to cover the range of melamine concentrations from 1 ng/mL to 1000 ng/mL (Figure 5) which is equivalent to approximately 50 ppb to 50 ppm in dry formula and 10 ppb to 10 ppm in liquid formula. The linearity of cyanuric acid in the method extended from 10 ng/mL to 1000 ng/mL (Figure 6) which is equivalent to approximately 250 ppb to 25 ppm in dry formula and 50 ppb to 5 ppm in liquid formula.
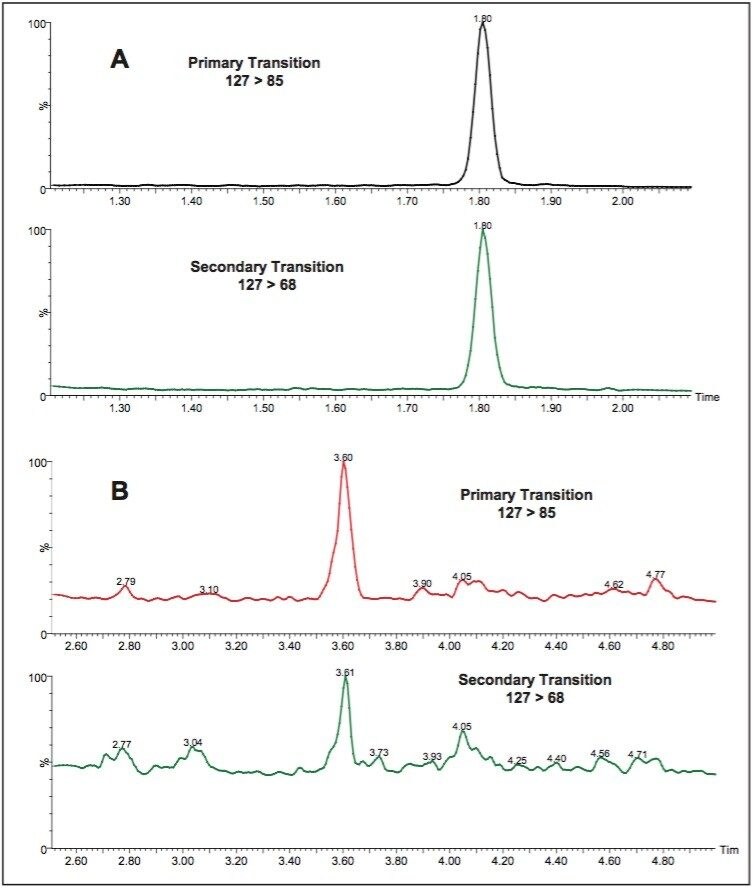
Taiwanese authorities have stated that melamine should not be detected in any foods. US FDA recently has stated that while a tolerance of 2.5 ppm may be applied to adult foods, melamine or one of its analogues alone below 1.0 ppm in infant formula do not raise public health concerns.7 This method was applied to the detection of melamine in liquid infant formula and demonstrated reliable detection and confirmation at the LOQ of 20 ppb on Atlantis HILIC Silica Column and 3 ppb on ACQUITY BEH HILIC Column (Figure 7). In the dry infant formula, the LOQ was 100 ppb on Atlantis HILIC Silica Column and 20 ppb on ACQUITY BEH HILIC Column.
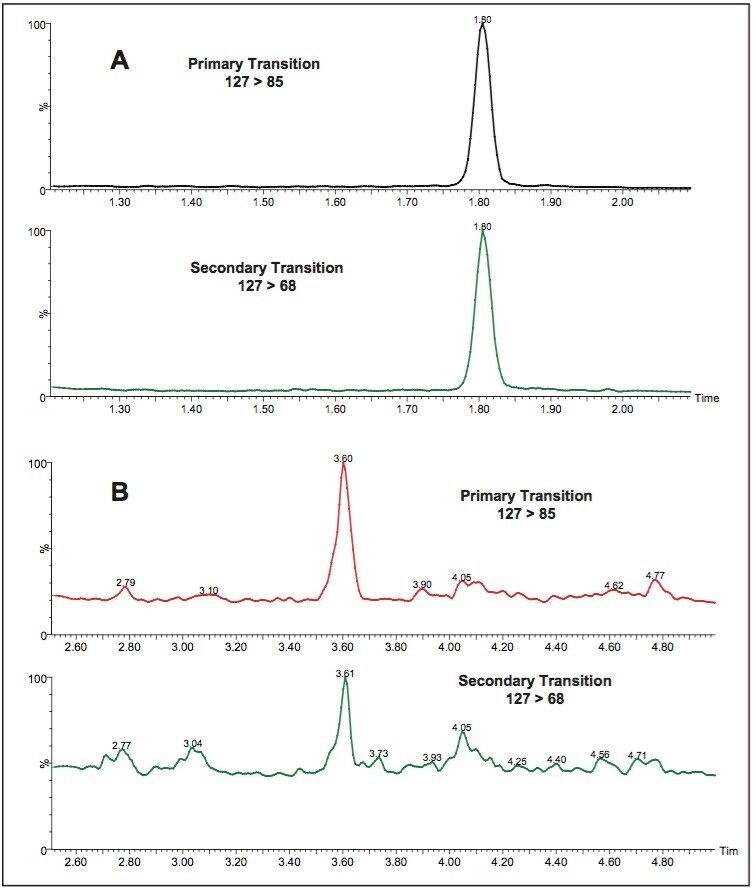
This method was also applied to the detection of cyanuric acid in liquid infant formula and demonstrated reliable detection and confirmation at the LOQ of 40 ppb on Atlantis HILIC Silica Column and 30 ppb on ACQUITY BEH HILIC Column (Figure 8). In the dry infant formula, the LOQ was 150 ppb on Atlantis HILIC Silica Column and 100 ppb on ACQUITY BEH HILIC Column.
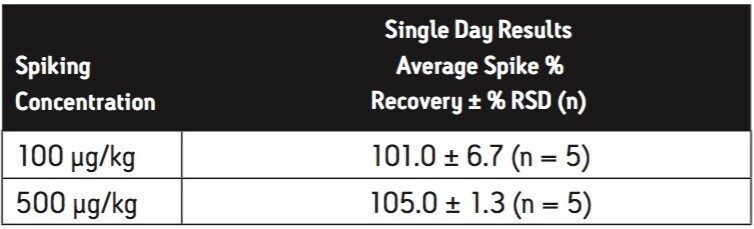
The spike recoveries of melamine and cyanuric acid at low concentrations are generally higher than those spiked at higher concentration. This may be due to the matrix interference or background from the reagents or environment. Due to the ubiquitous nature of melamine, it could be difficult to remove during trace analysis. Per the FDA interim method, the calibration standard solutions were prepared in the same elution solvent as during the solid-phase extraction. To account for the background/matrix interference, it may be necessary to calculate the concentration against calibration solutions prepared in a matrix-matched solvent, i.e. milk extract. To demonstrate such matrix effects, a series of calibration standards were prepared by fortifying the dry formula blank extract with melamine stock standards. All the samples and standards were analyzed using ACQUITY BEH HILIC Column. As shown in Table 13, the spike % recoveries are close to 100%, especially compared to the results without using matrixmatch calibration standards seen in Table 5.
There is a need for a rapid, sensitive method for the analysis of both melamine and cyanuric acid simultaneously in infant formula. This application note outlines the only complete single vendor solution for the quantitative analysis of melamine and cyanuric acid in infant formula. This note describes a method which uses 50:50 acetonitrile:water as the extraction solvent followed by two separate solid-phase extraction protocols for clean-up of the extracts: Oasis MCX for melamine and Oasis MAX for cyanuric acid. Two LC methods were developed on the ACQUITY UPLC System. The first HPLC method used an Atlantis HILIC Silica Column. The second method used an ACQUITY UPLC BEH HILIC Column coupled with the ACQUITY UPLC System gave a significantly reduced cycle time. The results are consistent with the published results indicated in the FDA interim method. The instrument analysis time using either method has been reduced compared to what listed in the method. The time saving is especially significant when using the ACQUITY UPLC BEH HILIC Column coupled with the ACQUITY UPLC System.
Tandem MS detection was carried out on the TQD which demonstrated not only excellent sensitivity, but good linearity across the wide range of concentrations required, when a stable isotope internal standard was added.
720002865, December 2008