Performance Comparison of ACQUITY™ Premier Fixed-Loop and ACQUITY UPLC™ I-Class PLUS Fixed-Loop Systems
Abstract
The ACQUITY Premier Fixed-Loop (FL) System is an Ultra Performance Liquid Chromatograph (UPLC) system which incorporates MaxPeak™ High Performance Surfaces (HPS) Technology with the FL injector to improve performance. The ACQUITY Premier FL System is positioned as a replacement for stainless-steel ACQUITY UPLC and ACQUITY UPLC I-Class PLUS FL systems. In this study, the chromatographic performance of metal-sensitive and non-metal-sensitive compounds on ACQUITY Premier FL and ACQUITY UPLC I-Class PLUS FL Systems is assessed using key performance characteristics such as peak tailing, peak height, area reproducibility, and retention time precision. The ACQUITY Premier FL System demonstrates improved performance for metal-sensitive analytes and comparable performance for non-metal-sensitive analytes compared to the ACQUITY UPLC I-Class PLUS FL System.
Benefits
- ACQUITY Premier Fixed-Loop (FL) System with MaxPeak High Performance Surfaces (HPS) Technology reduces metal interactions in the LC flow path
- ACQUITY Premier FL System demonstrates improved peak shape and peak area reproducibility for metal-sensitive analytes
- ACQUITY Premier FL System provides equivalent performance to ACQUITY UPLC I-Class PLUS FL for non-metal-sensitive targets
Introduction
Compounds that interact with metal surfaces present difficulties for analyses performed on stainless-steel LC systems. Metal-sensitive analytes can adsorb to exposed metal surfaces in the flow path, impacting chromatography and manifesting visually through increased peak tailing, reduced peak height, and poor peak area reproducibility.1–5 Common approaches to mitigate these metal interactions include using mobile phase additives, chemical passivation, and system priming or conditioning.2–4,6 While these approaches are successful in obtaining optimal system performance, they can be time intensive to deploy and often need to be repeated at regular intervals to maintain performance, ultimately affecting laboratory throughput.3,6
The ACQUITY Premier Fixed-Loop (FL) is an Ultra Performance Liquid Chromatograph (UPLC) with a FL injector engineered with MaxPeak High Performance Surfaces (HPS) Technology into its flow path to improve performance with metal-sensitive analytes. The fixed-loop is a common autosampler injector design, where a sample is aspirated into a sample loop before being injected onto the column.7 The FL has three injection modes—partial-loop with needle overfill, partial-loop, and full-loop—that can be selected based on the needs of the analysis. The FL design allows for fast cycle times and low extra-column dispersion performance as well as low sample consumption.7 The MaxPeak HPS Technology is an enhancement which provides a barrier that eliminates any potential interactions on the metal surfaces without the need for additives, passivation, or conditioning.4–5,8
In this study, the performance of ACQUITY Premier FL with MaxPeak HPS Technology and stainless-steel ACQUITY UPLC I-Class PLUS FL systems are compared to assess impact of system design on metal-sensitive and non-metal-sensitive analytes. Sample solutions containing metal-sensitive and non-metal-sensitive compounds were run on MaxPeak HPS and stainless-steel systems and key peak attributes were analyzed to evaluate chromatographic performance, including USP tailing factor, absolute peak height, peak area reproducibility, and retention time standard deviation.
Experimental
Sample Description
Hydrocortisone phosphate triethylamine was purchased from U.S. Pharmacopeia (USP) (Rockville, MD). Dexamethasone sodium phosphate, dexamethasone acetate, and dexamethasone were obtained from Sigma-Aldrich (St. Louis, MO). Individual stock solutions of each compound were prepared at 2 mg/mL in 50:50 Water/Acetonitrile.
Mixed sample solutions containing all four compounds (hydrocortisone phosphate, dexamethasone phosphate, dexamethasone acetate, and dexamethasone) were prepared at 1 µg/mL and 25 µg/mL (data not shown) in 50:50 Water/Acetonitrile.
Mobile Phases and Wash solvents were freshly prepared for each run.
LC Conditions
|
LC systems: |
1. ACQUITY Premier Fixed-Loop System with CH-A and BSM 2. ACQUITY UPLC I-Class PLUS Fixed-Loop System with CH-A |
|
Detection: |
1. ACQUITY UPLC PDA eλ Detector 2. ACQUITY UPLC PDA eλ Detector |
|
Sample: |
1 µg/mL mixed sample solution containing hydrocortisone phosphate, dexamethasone phosphate, dexamethasone acetate, and dexamethasone |
|
Columns: |
1. ACQUITY Premier BEH C18 Column 2.1 x 50 mm, 1.7 µm (p/n: 186009452) 2. ACQUITY UPLC BEH C18 Column 2.1 x 50 mm, 1.7 µm (p/n: 186002350) |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
10 µL (Full-loop injection mode) |
|
Flow rate: |
0.600 mL/min |
|
Mobile phase A: |
10 mM Ammonium Formate (pH 3.0) |
|
Mobile phase B: |
Acetonitrile |
|
Weak needle wash (WNW): |
90:10 Water/acetonitrile |
|
Strong needle wash (SNW): |
50:50 Water/acetonitrile |
|
Seal wash: |
90:10 Water/acetonitrile |
|
Wavelength: |
260 nm |
|
Collection rate: |
20 points/sec |
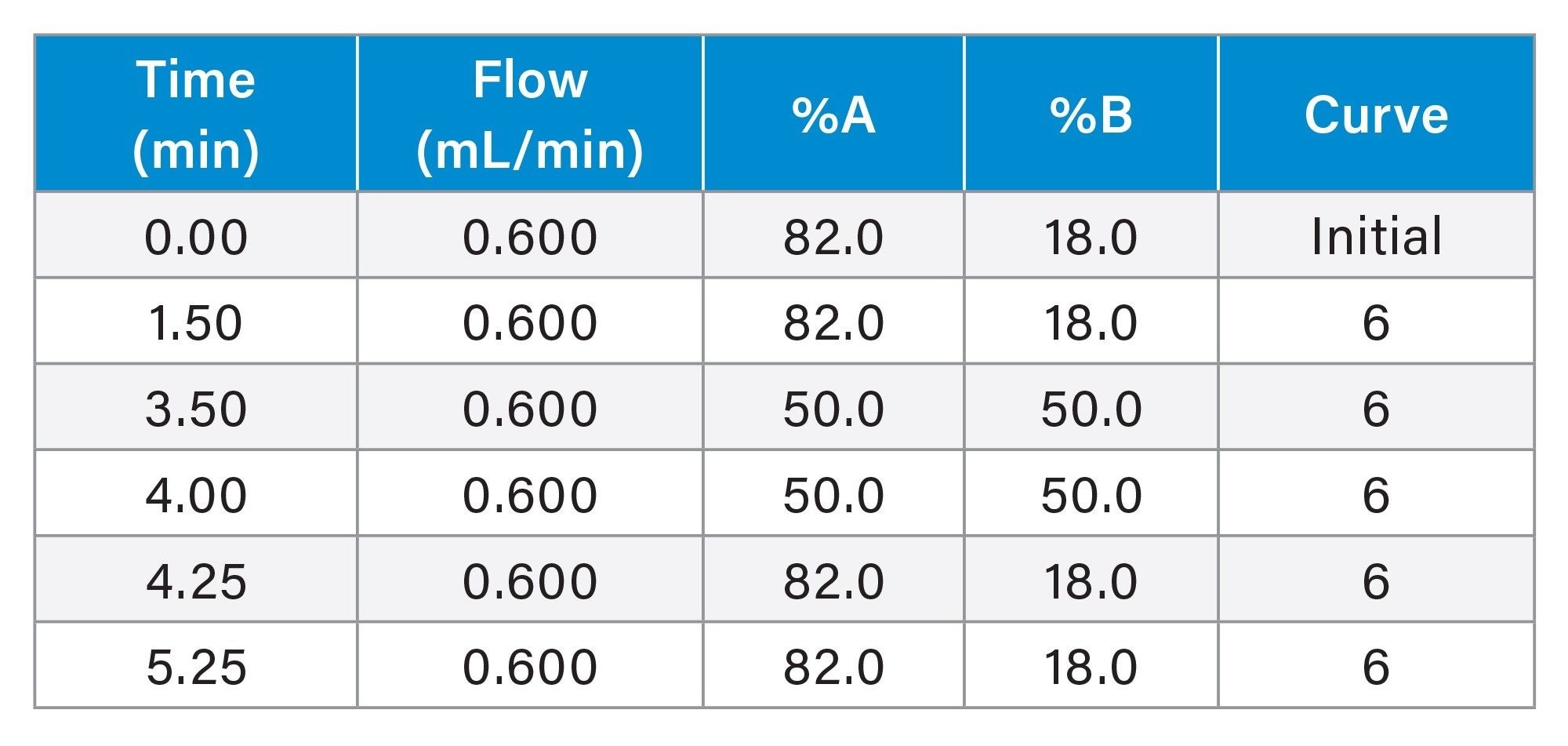
Gradient Table
Data Management
|
Software: |
Empower 3.6.1 |
Five ACQUITY Premier FL Systems and three ACQUITY UPLC I-Class PLUS FL Systems were used in this study. Common modules between the two system set-ups include CH-A column heaters and PDA eλ detectors. While the ACQUITY Premier FL System is available with either quaternary or binary solvent managers, the ACQUITY UPLC I-Class PLUS FL System is only available with a binary solvent manager. To match performance, ACQUITY Premier Binary Solvent Managers and ACQUITY UPLC I-Class Binary Solvent Managers were used for this study. ACQUITY Premier BEH C18 columns (p/n: 186009452) were installed on ACQUITY Premier FL Systems while stainless-steel ACQUITY UPLC BEH C18 columns (p/n: 186002350) were installed on ACQUITY UPLC I-Class PLUS FL Systems.
Results and Discussion
In this study, the chromatographic performance of ACQUITY Premier FL and ACQUITY UPLC I-Class PLUS FL Systems were assessed. In stainless-steel LC systems, such as the ACQUITY UPLC I-Class PLUS FL, metal-sensitive analytes can adsorb to exposed metal surfaces in the flow path through the Lewis acid-base reaction, as described by Lauber et al. (2021). On metal surfaces, there is often a thin layer of metal oxide. The metal ions in this layer are electron deficient and act as Lewis acids. Metal-sensitive compounds often have electron-rich moieties, such as phosphate and carboxylate groups, and act as Lewis bases. These attributes allow metal-sensitive compounds to bind with metal ions on the surfaces of a stainless-steel LC flow path. This interaction manifests visually in chromatography through increased peak tailing, reduced absolute peak height, and decreased peak area reproducibility. The MaxPeak HPS Technology of ACQUITY Premier FL System mitigates interactions with metal surfaces, minimizing band broadening as analytes pass through the system.
To compare the performance of ACQUITY Premier FL and ACQUITY UPLC I-Class PLUS FL Systems, six sample sets consisting of six replicate injections of mixed sample solutions with metal-sensitive and non-metal-sensitive compounds were run on reversed-phase C18 columns. Figure 1 shows representative chromatograms of the four target peaks analyzed in the samples. The metal-sensitive compounds are hydrocortisone phosphate (HCP; labeled as Peak 1) and dexamethasone phosphate (DMP; Peak 2). These compounds contain electron-rich phosphate groups that will bind to metal ions on the exposed metal surface. The non-metal-sensitive compounds are dexamethasone (Peak 3) and dexamethasone acetate (DAC; Peak 4). These compounds are used to determine comparable performance.
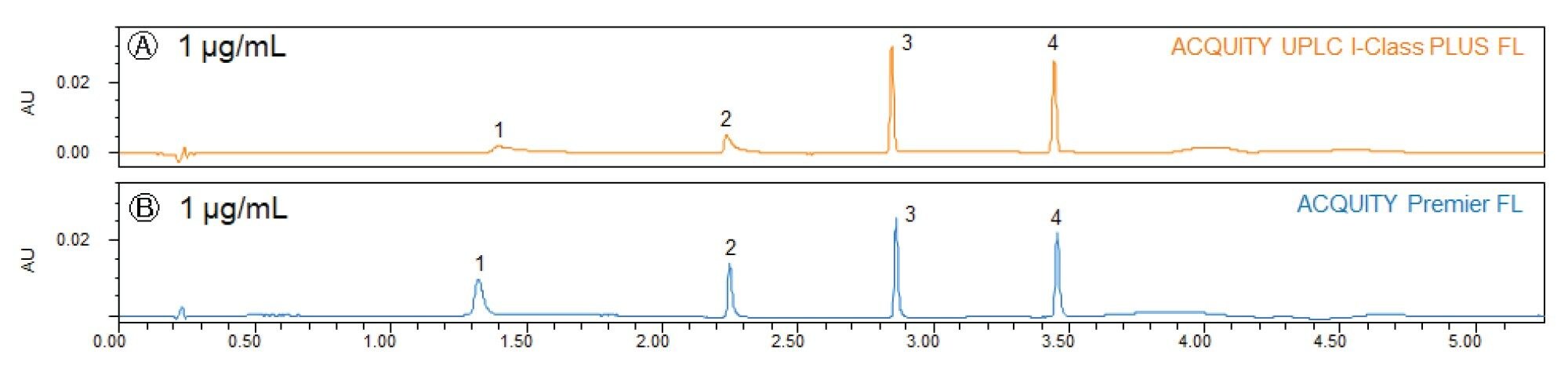
Two concentration levels, 1 µg/mL and 25 µg/mL (data not shown), were initially selected to examine the effects of conditioning on the systems. A conventional LC system is primed or conditioned when exposed active sites in the flow path are blocked and system performance is restored. This conditioning effect was well observed in the 25 µg/mL samples, where the abundance of metal-sensitive analytes competing for available active sites effectively primed the stainless-steel systems and minimized the performance disparity between the stainless-steel and MaxPeak HPS systems. Conditioning was not sufficient to restore system performance in the 1 µg/mL samples because fewer active sites are blocked and, therefore, the effects of metal adsorption are more pronounced in the chromatography. As such, this study will focus on low concentration samples where conditioning is not an effective countermeasure for metal adsorption.
Improved Performance: Metal-Sensitive Analytes
Due to metal interactions in a stainless-steel flow path, the peak shape of a metal-sensitive analyte is distorted. A metal-sensitive compound that adsorbs to exposed metal surfaces will be more asymmetrical, showing an elongated peak with a long tail. Since the peak takes longer to elute, the amount of compound passing through the detector is lower over the elution duration, resulting in a reduction of peak height.
This phenomenon is visualized in Figure 2, where due to interactions with metal surfaces of the ACQUITY UPLC I-Class PLUS FL System, HCP and DMP elute as asymmetrical peaks with long tails and reduced peak heights. MaxPeak HPS Technology is designed to prevent these peak shape distortions by eliminating any active sites in the flow path to which metals can bind, keeping peaks sharp and symmetrical. On the ACQUITY Premier FL System with MaxPeak HPS Technology, these metal-sensitive compounds are protected from metal interactions, resulting in taller peaks that show less tailing. Three peak attributes are used as proxies to assess metal-sensitivity: peak tailing, peak height, and peak area reproducibility.
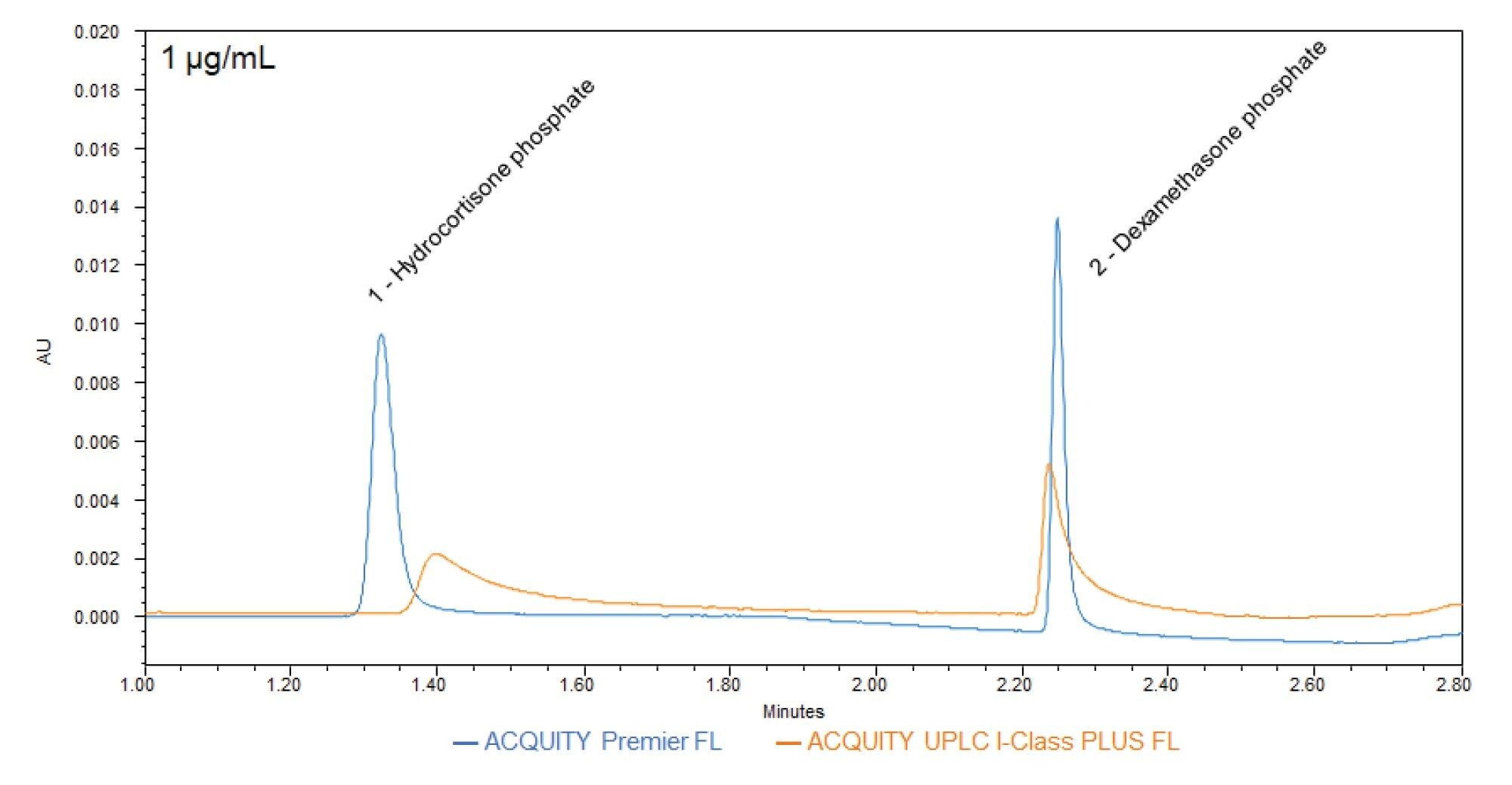
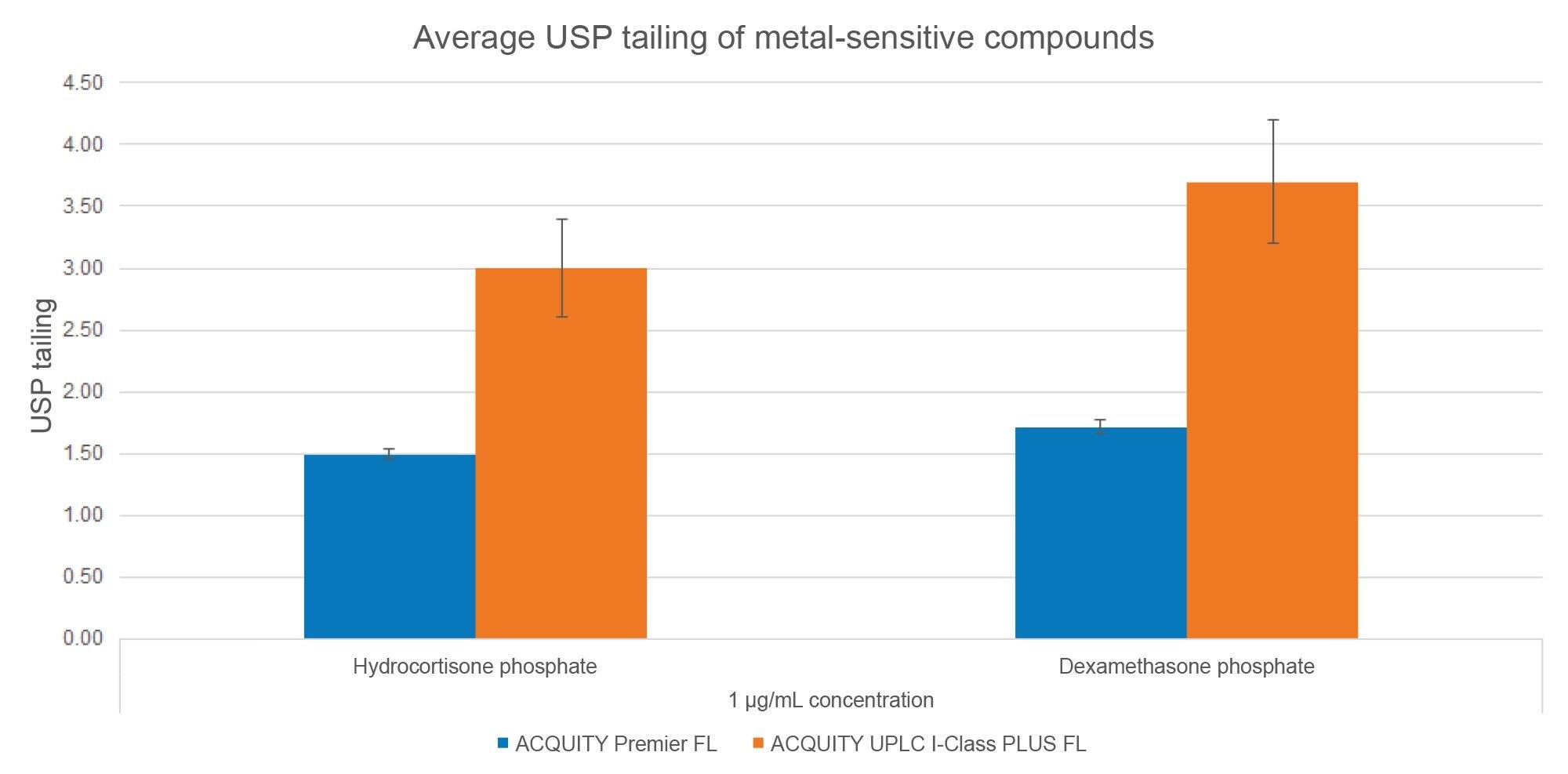
To analyze peak shape, USP tailing factor and absolute height of HCP and DMP were determined for the sample sets (Figures 3 and 4, respectively). USP tailing factor measures the asymmetry of a peak, with a value of 1 corresponding to a perfectly symmetrical peak. The average USP tailing factors of HCP and DMP are lower on the MaxPeak HPS systems than on the stainless-steel systems (Figure 3). The average absolute heights of HCP and DMP are greater on the MaxPeak HPS systems than on the stainless-steel systems (Figure 4). Both factors indicate that fewer metal interactions are occurring in the flow path of the ACQUITY Premier FL Systems.
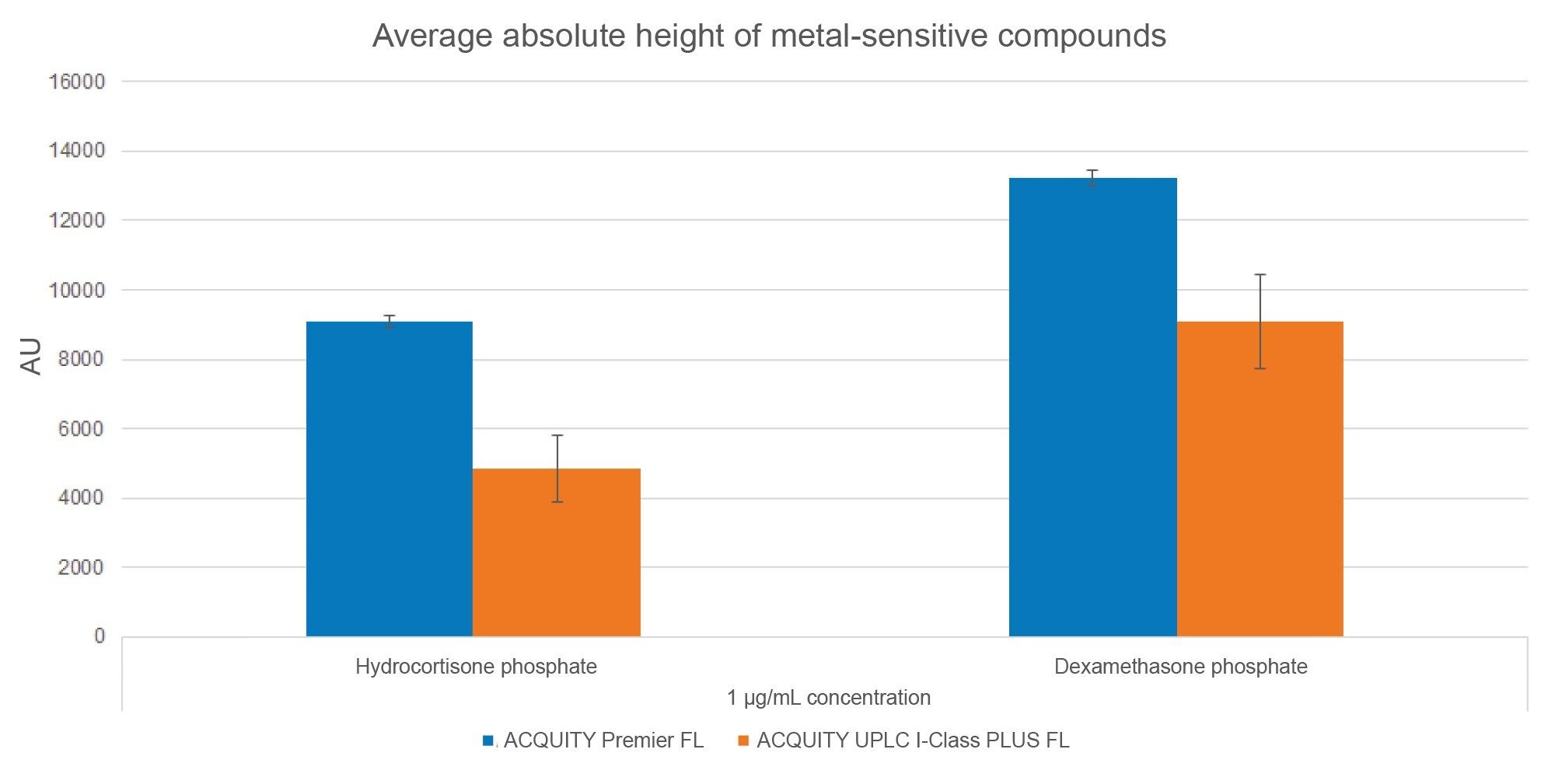
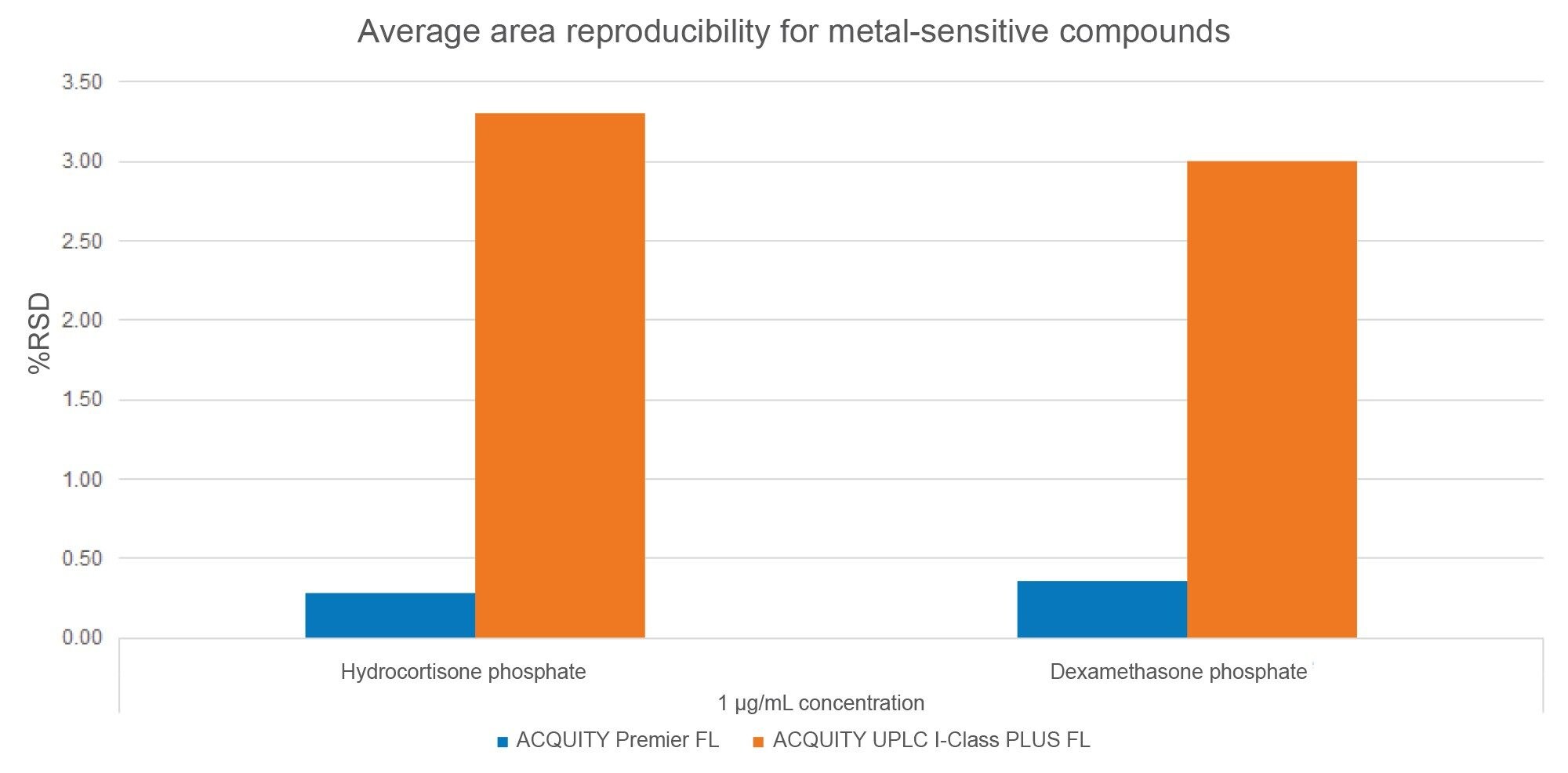
In addition to peak shape, peak area reproducibility is a proxy to assess metal sensitivity. Until a system is fully conditioned, metal-sensitive compounds often show poor reproducibility among injections, with wide variations in peak height and peak area. An analyte with poor area reproducibility has a large peak area % relative standard deviation (RSD). This is demonstrated in Figure 5 for HCP and DMP using average peak area %RSD. The average peak area %RSD values of the compounds are below 0.5% on the ACQUITY Premier FL systems but exceed 2.9% on the ACQUITY UPLC I-Class PLUS FL systems. The good peak reproducibility on the ACQUITY Premier FL systems indicates that fewer metal interactions are taking place.
Comparable Performance: Non-Metal-Sensitive Analytes
In contrast, non-metal-sensitive analytes minimally interact with metal surfaces in the flow path. Therefore, these compounds can be used to assess comparable performance between ACQUITY Premier FL and ACQUITY UPLC I-Class PLUS FL systems, since the MaxPeak HPS enhancement is not a differentiating factor. For this comparison, peak area reproducibility and retention time standard deviation are analyzed as a measure of consistent performance for non-metal-sensitive compounds, dexamethasone and dexamethasone acetate.
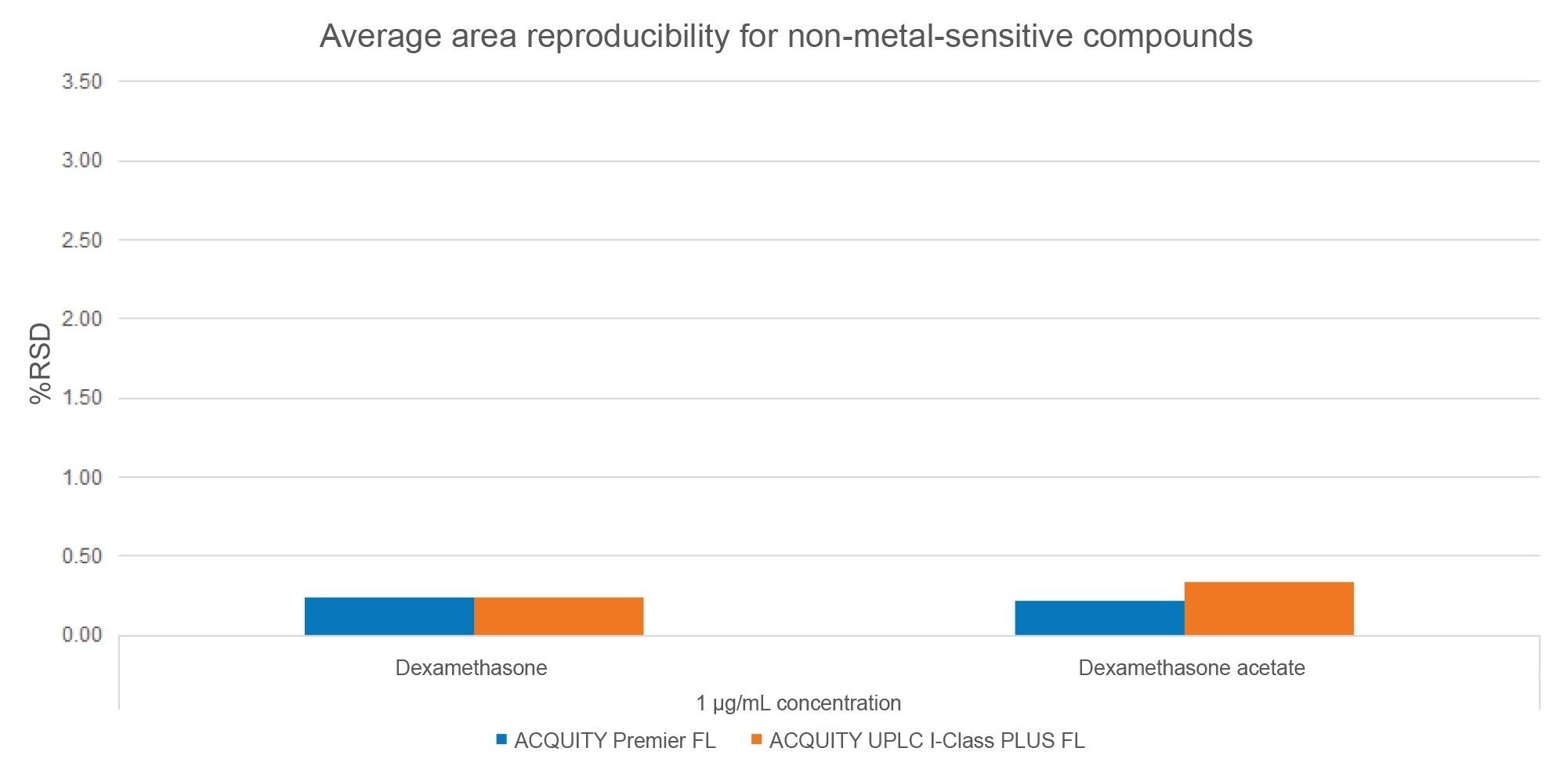
As previously discussed, poor peak area reproducibility is an indicator of metal interactions. Non-metal-sensitive compounds are not subject to these interactions. To this point, as seen in Figure 6, the non-metal-sensitive compounds demonstrated good area reproducibility with low peak area %RSD values across all systems (%RSD<0.5%). No significant performance disparity was observed between the MaxPeak HPS and the stainless-steel systems.
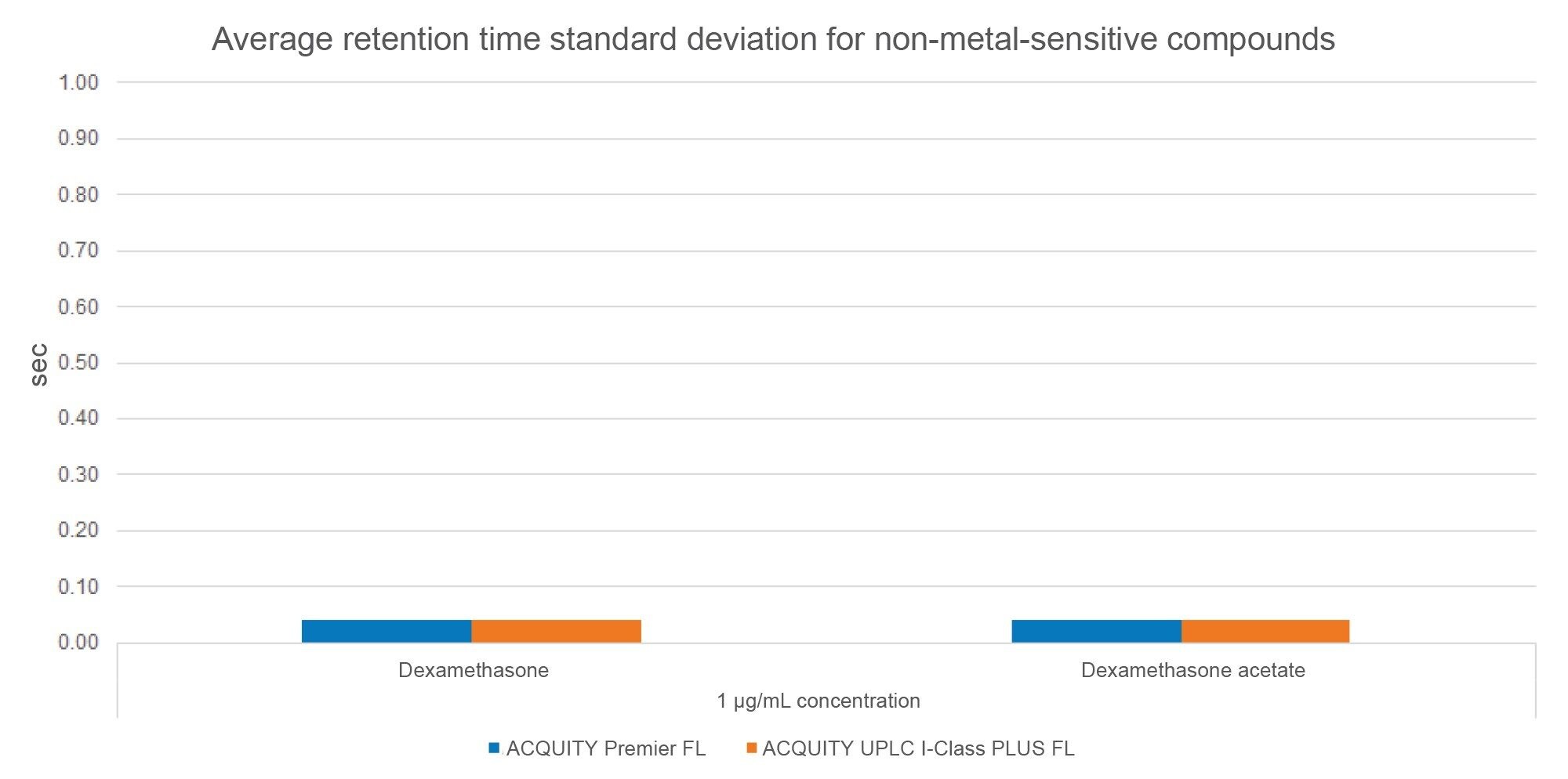
Another metric assessed in this study is retention time reproducibility, which is a measure of consistent performance. Retention times of metal-sensitive analytes often shift over time as stainless-steel systems become conditioned. Conversely, non-metal-sensitive analytes that minimally interact with metal surfaces would not exhibit this retention time shift. As seen in Figure 7, the average retention time standard deviations (SD) for dexamethasone and DAC were equivalent across the systems with a value of 0.040 seconds, indicating no significant performance disparity between the MaxPeak HPS and stainless-steel systems.
Performance Summary
Overall, ACQUITY Premier FL demonstrated better chromatographic performance for metal-sensitive analytes and comparable performance for non-metal-sensitive analytes. Metal-sensitive analytes HCP and DMP showed improved peak shape and peak area reproducibility on the MaxPeak HPS systems. Non-metal-sensitive analytes dexamethasone and DAC showed no significant performance disparity between the systems for peak area reproducibility and retention time SD. Taken together, these results show that ACQUITY Premier FL outperforms ACQUITY UPLC I-Class PLUS FL for metal-sensitive targets and demonstrates equivalent chromatographic performance for non-metal-sensitive targets.
Conclusion
Metal-sensitive compounds are a challenging analysis for conventional stainless-steel LC systems, often requiring intensive and time-consuming preparations of mobile phase additives, system passivation, or conditioning for optimal performance, which can affect throughput. Due to metal interactions in the flow path, these compounds often exhibit poor chromatography, which may include distorted peak shapes such as increased peak tailing and reduced peak height and result in lower detector responses and poor peak area reproducibility. In this study, the chromatographic performance of ACQUITY Premier FL with MaxPeak HPS Technology was compared to the stainless-steel ACQUITY UPLC I-Class PLUS FL. For metal-sensitive analytes, the ACQUITY Premier FL consistently demonstrated reduced peak tailing, greater peak height, and better peak area reproducibility without the need for system conditioning. In addition, for non-metal-sensitive analytes, the ACQUITY Premier FL provided equivalent performance to the ACQUITY UPLC I-Class PLUS FL with respect to peak area reproducibility and retention time standard deviation. The ACQUITY Premier FL with MaxPeak HPS Technology is a versatile and convenient solution to the problem of metal-sensitive compounds without sacrificing performance for non-metal-sensitive compounds.
References
- Gilar, M.; DeLano, M.; Gritti, F. Mitigation of analyte loss on metal surfaces in liquid chromatography. Journal of Chromatography A, Volume 1650, August 2021, 462247. https://doi.org/10.1016/j.chroma.2021.462247
- Lauber, M.; Walter, T.; Gilar, M.; DeLano, M.; Boissel, C.; Smith, K.; Birdsall, R.; Rainville, P.; Belanger, J.; Wyndham, K. Low Adsorption HPLC Columns Based on MaxPeak High Performance Surfaces (HPS). Waters White Paper, 720006930, 2021.
- Martin, W.; Shah, D.; Grzonka, C.; Dovell, A.; Li, Z.; Hong, P.; Turyan, I.; Dyke, J. Recovery of Metal-Sensitive Analytes on the Arc Premier Solution: System-to-System Reproducibility and a Multi-System Comparison to Conventional LC. Waters Application Note, 720007330, August 2021.
- Li, Z.; Shah, D.; Dovell, A.; Martin, W., Grzonka, C.; Dyke, J. Challenge Accepted: Arc Premier System Increases Sensitivity and Reproducibility for Hard-to-See Compounds. Waters Application Note, 720007267, June 2021.
- Patel, A.; Simeone, J.; Delano, M.; Dyke, J.; Rzewuski, S.; Jung, M.; Shiner, S. Waters Premier Standards to Investigate the Inertness of Chromatographic Surfaces. Waters Application Note, 720007105, March 2021.
- Koshel, B.; Simeone, J.; Dao, D.; Nguyen, J.; Rzewuski, S.; Lauber, M.; Birdsall, R.; Yu, Y. Bypassing LC System Passivation Requirements Using ACQUITY Premier with MaxPeak HPS Technology for the Recovery of a Phosphorylated Peptide. Waters Application Note, 720006921, May 2020.
- McConville, P. Enhanced Sample Delivery Mechanism for Ultra Low Dispersion and Carryover Performance. Waters Application Note, 720003443, April 2018.
- Tanna, N.; Plumb, R.; Mullin, L. Improvements in Sensitivity for Quantification of Steroid Phosphate Drugs Using ACQUITY Premier System and ACQUITY Premier Columns. Waters Application Note, 720007095, February 2021.
720007899, May 2023