For research use only. Not for use in diagnostic procedures.
The aim of this study was to compare the performance and benefits of automated sample preparation using a Tecan Freedom EVO 100 liquid handler to manual sample preparation in the context of a routine clinical research application. For the determination of a panel of 21 opioids in human urine by solid-phase extraction (SPE) LC-MS/MS, manual and automated sample preparation runs were performed on each of three days to compare linearity, precision, accuracy, carryover, and sample preparation time.
Automated sample preparation improves laboratory operations by a) reducing errors in sample tracking and preparation, b) producing more consistent results free of analyst-to-analyst variation, c) allowing analysts to work more efficiently, and d) minimizing laboratory hazards in regard to solvent exposure and repetitive motions associated with manual pipetting. For labs considering automation, the aim of this study was to compare the performance and benefits of automated sample preparation using a Tecan Freedom EVO 100 liquid handler to manual sample preparation in the context of a routine clinical research application. For the determination of a panel of 21 opioids in human urine by solid-phase extraction (SPE) LC-MS/MS, manual and automated sample preparation runs were performed on each of three days to compare linearity, precision, accuracy, carryover, and sample preparation time.

All analytes and internal standards were purchased from Cerilliant (Round Rock, TX). Surine XTD was purchased from Dyna-Tek Industries (Shawnee Mission, KS). A combined analyte stock solution was prepared in blank human urine (1000 ng/mL, 200 ng/mL fentanyl–norfentanyl).
A combined internal standard stock solution was prepared in methanol and an internal standard working solution was prepared in Surine. Corresponding deuterated internal standards were used for all analytes except hydromorphone-3-β-D-glucuronide, which used morphine-3-β-D-glucuronide-D3 as an internal standard. Calibrators and QCs were prepared in human urine. Calibrators were prepared at six levels from 20–1000 ng/mL (4–200 ng/mL for fentanyl–norfentanyl); QCs were prepared at 30, 150, and 750 ng/mL (6, 30, and 150 ng/mL for fentanyl– norfentanyl). Calibrators and QCs were split for the automated and manual sample preparations.
A robust solid-phase extraction (SPE) sample preparation method was developed for 21 opiate/opioid drugs and metabolites (see Table 1). An enzymatic hydrolysis step was not included in the method; rather, glucuronides were included as analytes. The following procedure was used for both automated and manual sample preparation.
Urine samples (150 μL) were combined with 50 μL of internal standard and 200 μL of 4% phosphoric acid in a 2 mL mixing plate. For extraction, samples were transferred to an Oasis MCX μElution 96-well plate and eluted into a 1 mL collection plate. The SPE procedure was as follows:
|
Condition: |
200 μL MeOH |
|
Equilibrate: |
200 μL H2O |
|
Sample load: |
375 μL |
|
Wash 1: |
200 μL H2O |
|
Wash 2: |
200 μL MeOH |
|
Elution (2x): |
50 μL of 5% NH4OH in 60:40 MeOH–ACN |
The eluted samples were blown down to dryness using a nitrogen evaporator and reconstituted in 50 μL of 2% formic acid in 98:2 water–acetonitrile before shaking for ten minutes.
The manual sample preparations were performed by an experienced analyst. A calibrated multichannel pipette was used throughout the extraction.
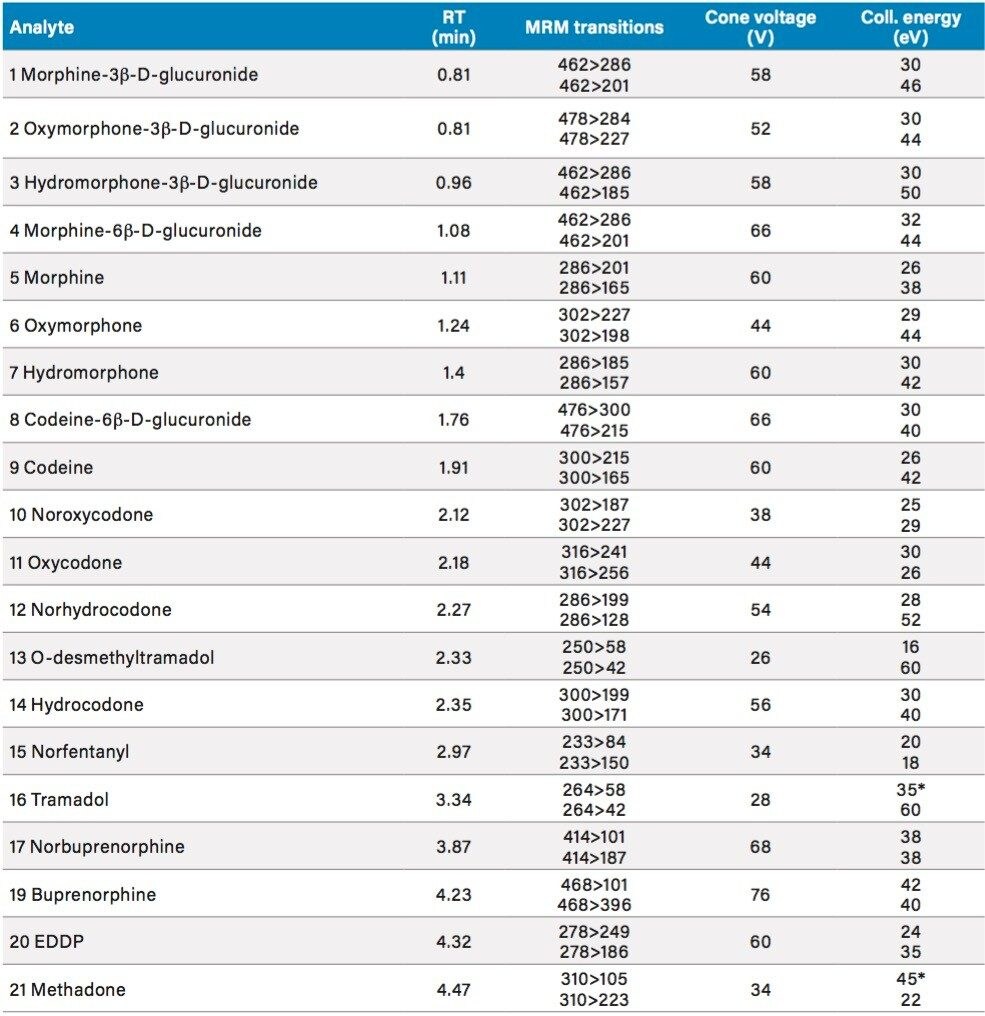
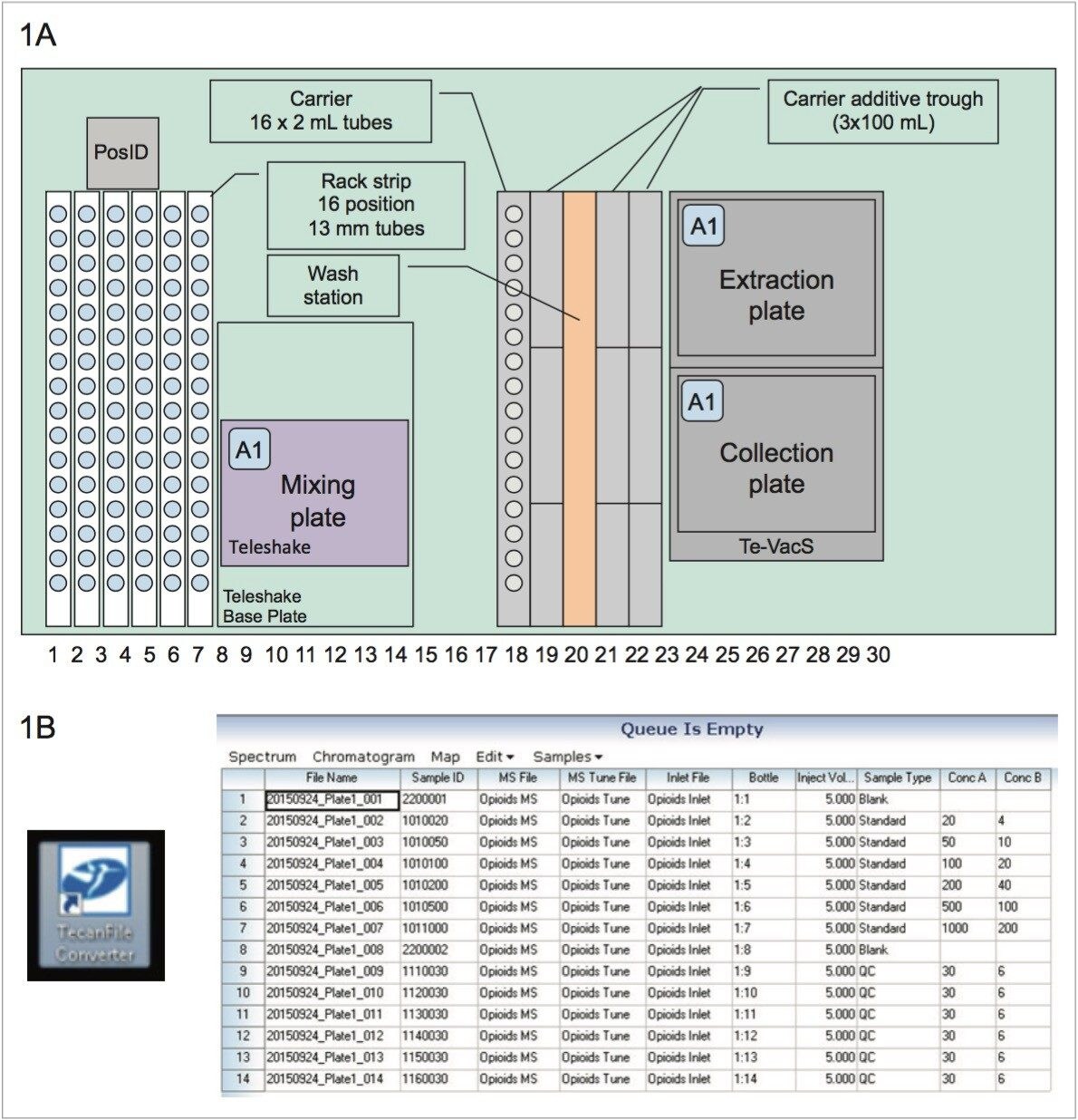
The Tecan Freedom EVO 100 liquid handler has a user-configurable worktable and components to automate a variety of sample preparation operations. For this study, the liquid handler was equipped with sample and internal standard tube racks, reagent racks and troughs, 4-tip liquid handling arm for sample transfers and reagent additions, robotic manipulator arm for moving plates, bar code reader (posID), plate shaker (Teleshake), wash station, and vacuum manifold (Te-VacS). Pipetting tips were fixed (i.e., non-disposable) and were washed between transfers with the vendor-recommended solution of 5% isopropanol in water. The liquid handler executed the extraction as specified by the software script. Upon completion of the script, the Tecan MassLynx File Converter software automatically created a sample list with specimen IDs, plate locations, and pre populated method information for import into MassLynx via .csv file. The combined use of automated sample preparation with the file converter provides sample traceability from the sample tube through the completion of the LC MS/MS analysis, thereby reducing the potential for sample mix-ups as well as errors associated with sample preparation and sample information transcription.
|
LC system: |
ACQUITY UPLC |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 mm x 100 mm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Mobile phase A: |
H2O with 0.1% formic acid |
|
Mobile phase B: |
ACN with 0.1% formic acid |
|
Weak needle wash: |
2% ACN in H2O |
|
Strong needle wash: |
ACN |
|
Time (min) |
Flow rate (min) |
%A |
%B |
|---|---|---|---|
|
0.00 |
0.6 |
98 |
2 |
|
3.00 |
0.6 |
80 |
20 |
|
4.00 |
0.6 |
55 |
45 |
|
4.10 |
0.6 |
90 |
10 |
|
4.60 |
0.6 |
90 |
10 |
|
4.70 |
0.6 |
98 |
2 |
|
6.20 |
0.6 |
98 |
2 |
|
Injection volume: |
5 μL |
|
MS system: |
Xevo TQD Mass Spectrometer |
|
Ionization mode: |
ESI+ |
|
Acquisition mode: |
MRM (see Table 1 for transitions) |
|
Capillary voltage: |
0.5 kV |
|
Cone voltage (V): |
Optimized for each analyte |
|
Collision energy (eV): |
Optimized for each analyte |
Data were acquired and processed using MassLynx v4.1 Software. Quantification was performed using TargetLynx Application Manager.
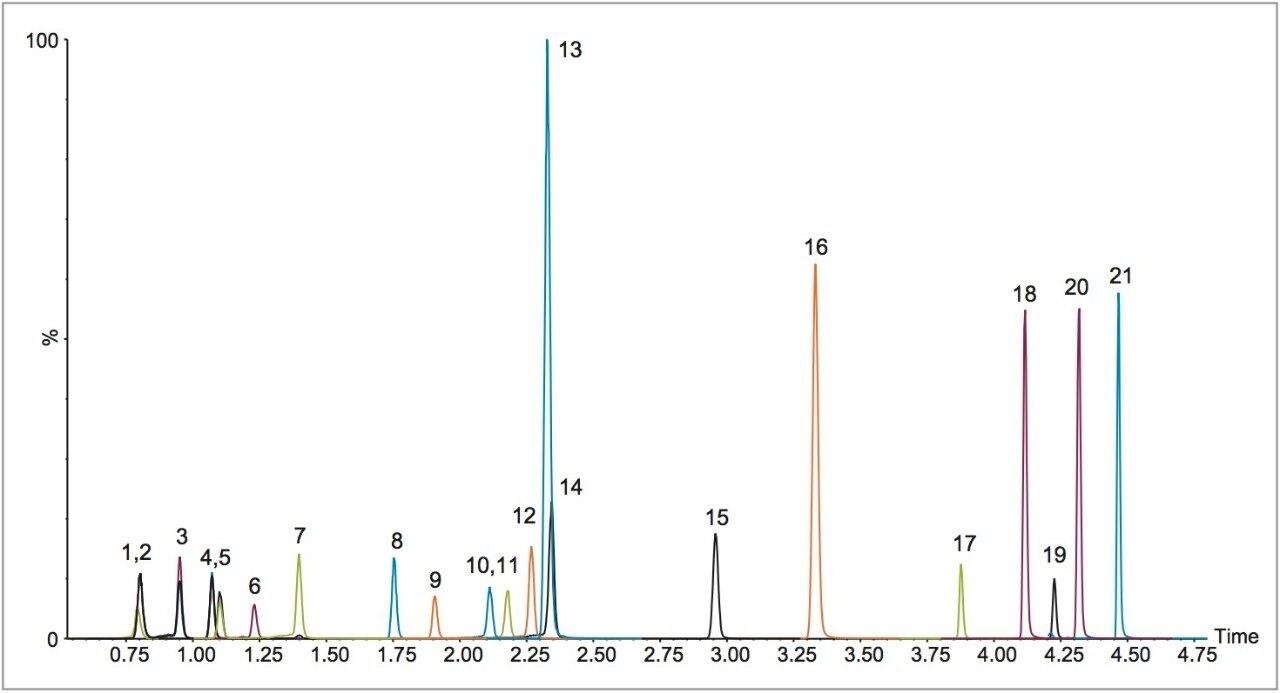
Manual and automated sample preparation LC-MS/MS runs were performed on each of three days to compare linearity, inter-assay precision and accuracy, carryover, and sample preparation time. Plates from manual and automated sample preparation each included blank samples, duplicate bracketing calibrators at six levels from 20–1000 ng/mL (4–200 ng/mL fentanyl–norfentanyl), and three levels of QCs (n=6/level) at 30, 150, and 750 ng/mL (6, 30, and 150 ng/mL fentanyl–norfentanyl). Results are summarized in Tables 3–5.
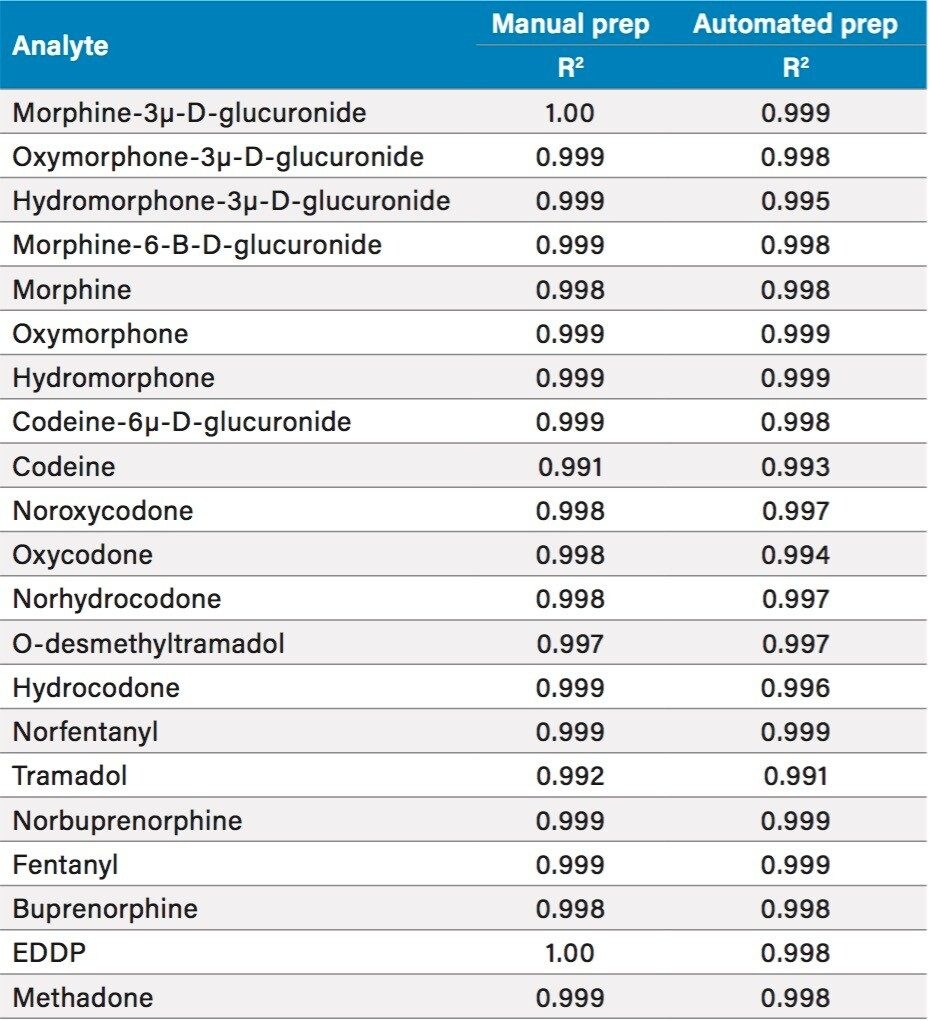
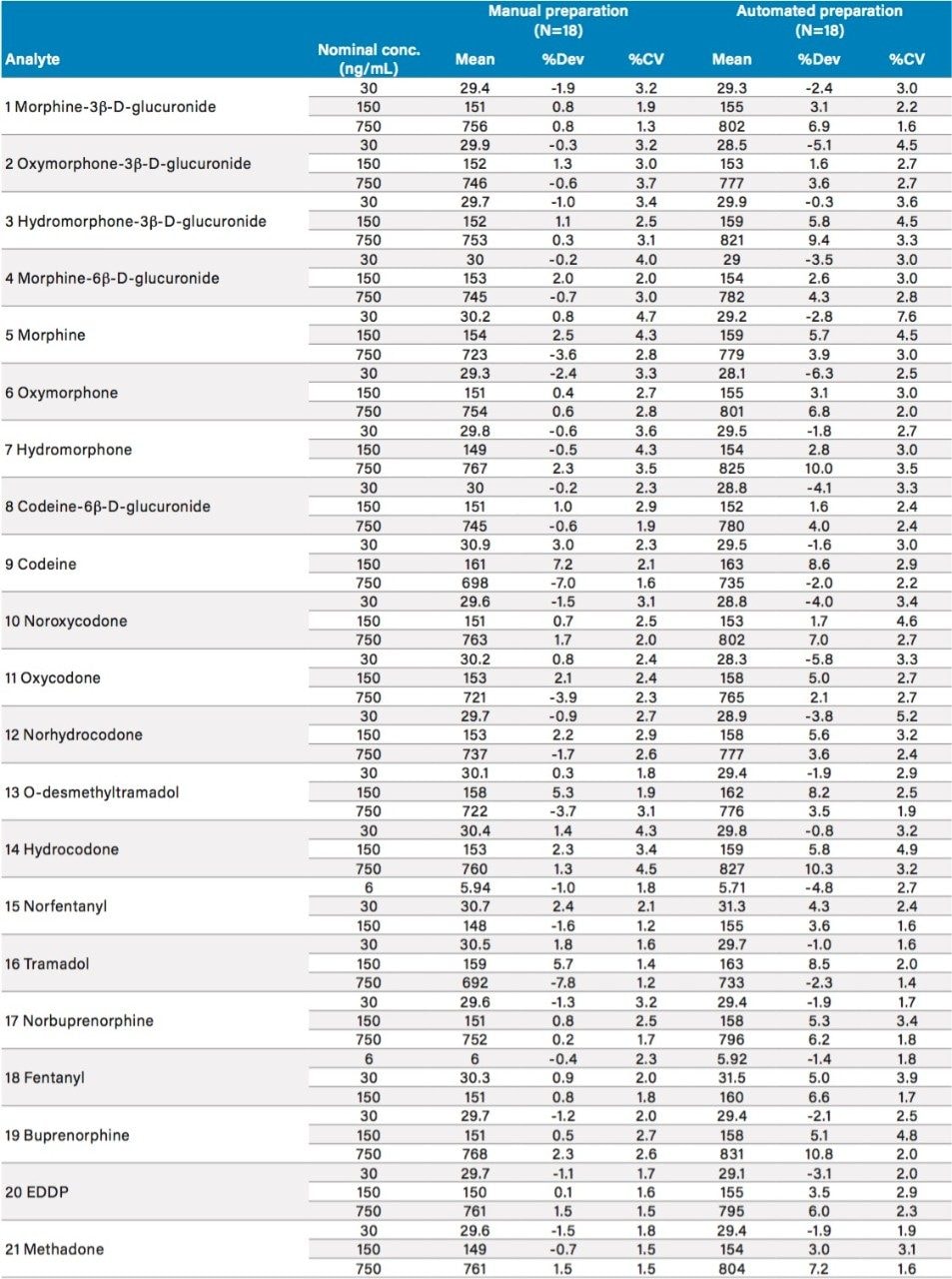
Both types of sample preparation produced linearity, precision, and accuracy results that met industry-standard acceptance criteria; in many cases, interassay means and variance were not statistically different (t-test and F-test). For both types of sample preparation, carryover – evaluated by comparing the mean analyte response from the blanks injected after the highest standard (n=2) to the mean response from the lowest standard (n=2) – was less than 4% for all 21 analytes.
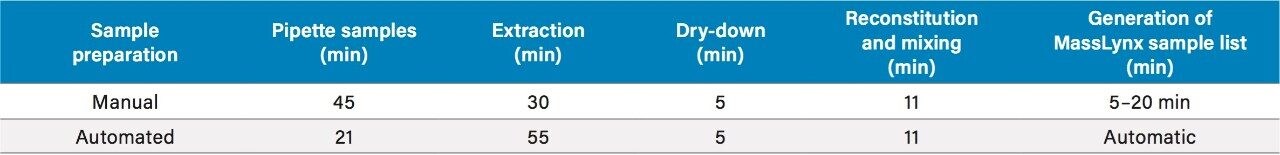
Sample processing time for the manual and automated approaches did not differ significantly. However, the use of the Tecan MassLynx File Converter to generate MassLynx sample lists saved considerable amounts of time in the overall analysis, while minimizing transcription errors.
Automated sample preparation produced results similar, and in many cases statistically equivalent to, manual sample preparation. The time required for automated sample preparation was also similar to that required for manual preparation. However, automated sample preparation was overall faster when the Tecan MassLynx File Converter was used to automatically generate an importable MassLynx sample list. Automated sample preparation has the additional benefits of allowing analysts to spend more time on tasks requiring human intervention while also reducing the potential for variation and error at multiple points during sample preparation and analysis. The Oasis MCX μElution Plate provides identical results when used in either manual or automated sample preparation procedures. Finally, the combination of the sample-tracking capabilities of the Tecan liquid handler with the Tecan MassLynx File Converter software can reduce transcription errors.
720005849, March 2017