This is an Application Brief and does not contain a detailed Experimental section.
This application brief confirm and quantify the presence of macrolides and other important classes of antibiotics in waste water treatment plant (WWTP) effluent and surface waters at low ppt levels.
This method has undergone full validation according to the method previously published by the U.S. EPA.
The presence of macrolides such as clarithromycin in WWTP effluent waters poses a potential ecotoxicological risk. Additionally the general occurrence of antibiotics in treated wastewater is concerning due to the probable development of antibiotic resistance among bacteria in nature. In the latest update from the EU Commission Implementing Decision (EU) 2015/495, a watch list of substances for EU-wide monitoring in the field of water policy was established.¹ Compounds of interest include the three macrolides azithromycin, clarithromycin, and erythromycin, requiring a maximum method detection limit of 90 ng/L by LC-MS/MS. These were included to gather additional data concerning their presence in aquatic environments and the possible risks associated thereby. In this application brief, we describe the analysis of azithromycin, clarithromycin, and erythromycin in WWTP effluent and surface waters, along with three additional important classes of antibiotics, utilizing off-line solid phase extraction followed by analysis on a Waters ACQUITY UPLC H-Class System equipped with a column manager, coupled to a Xevo TQ-S Mass Spectrometer.
WWTP effluent and surface water samples were extracted by means of an optimized solid-phase extraction methodology using 200 mg Oasis HLB Solid Phase Extraction (SPE) Cartridges.² Prior to extraction, the cartridges were conditioned with methanol (MeOH) and reagent water. Samples were passed through the SPE cartridges followed by air drying under a positive pressure, a step that proved crucial for both recovery results and evaporation time reproducibility. The extracts were eluted with MeOH into disposable borosilicate glass tubes and evaporated to complete dryness. The dry extracts were reconstituted to a total volume of 1 mL in TruView LC-MS Certified Vials. Finally 1 μL of this sample was injected onto the ACQUITY UPLC H-Class System equipped with a column manager, enabling fast column switching between two ACQUITY UPLC BEH C18 Columns (2.1 mm x 50 mm, 1.7 μm) in parallel. The columns were run using identical simple linear gradients, one column ran under basic conditions and the other acidic conditions. It has been shown that substantial selectivity and sensitivity gains can be achieved by placing each compound at a suitable column pH without prejudices regarding historical residence in terms of chromatographic conditions and ESI mode.³ This increases the method’s flexibility so it can be implemented in routine analysis in response to new requests as well as for contaminants yet to be discovered.
The antibiotics analyzed and the UPLC conditions are described in Table 1. The samples were determined using electrospray in positive ion mode with two MRM transitions per compound. Figure 1 shows the chromatograms obtained for the three macrolides, while Figure 2 demonstrates linearity showing the calibration curve for clarithromycin. The linearity, method quantification limit (MQL), absolute SPE recovery, and method recovery for all of the investigated antibiotics are detailed in Table 1. The MQL for all macrolides is at the very low ppt level for the complete SPE methodology. The developed procedure has undergone full validation according to the method previously published by the U.S. Environmental Protection Agency.⁴
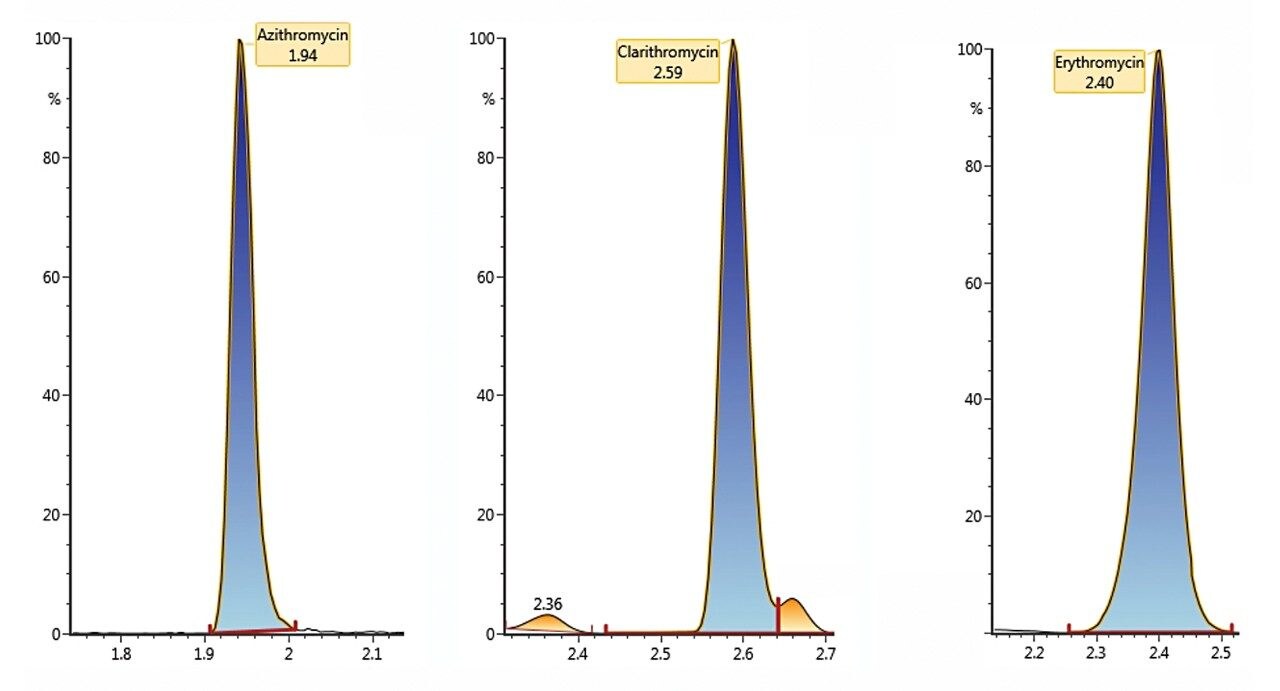

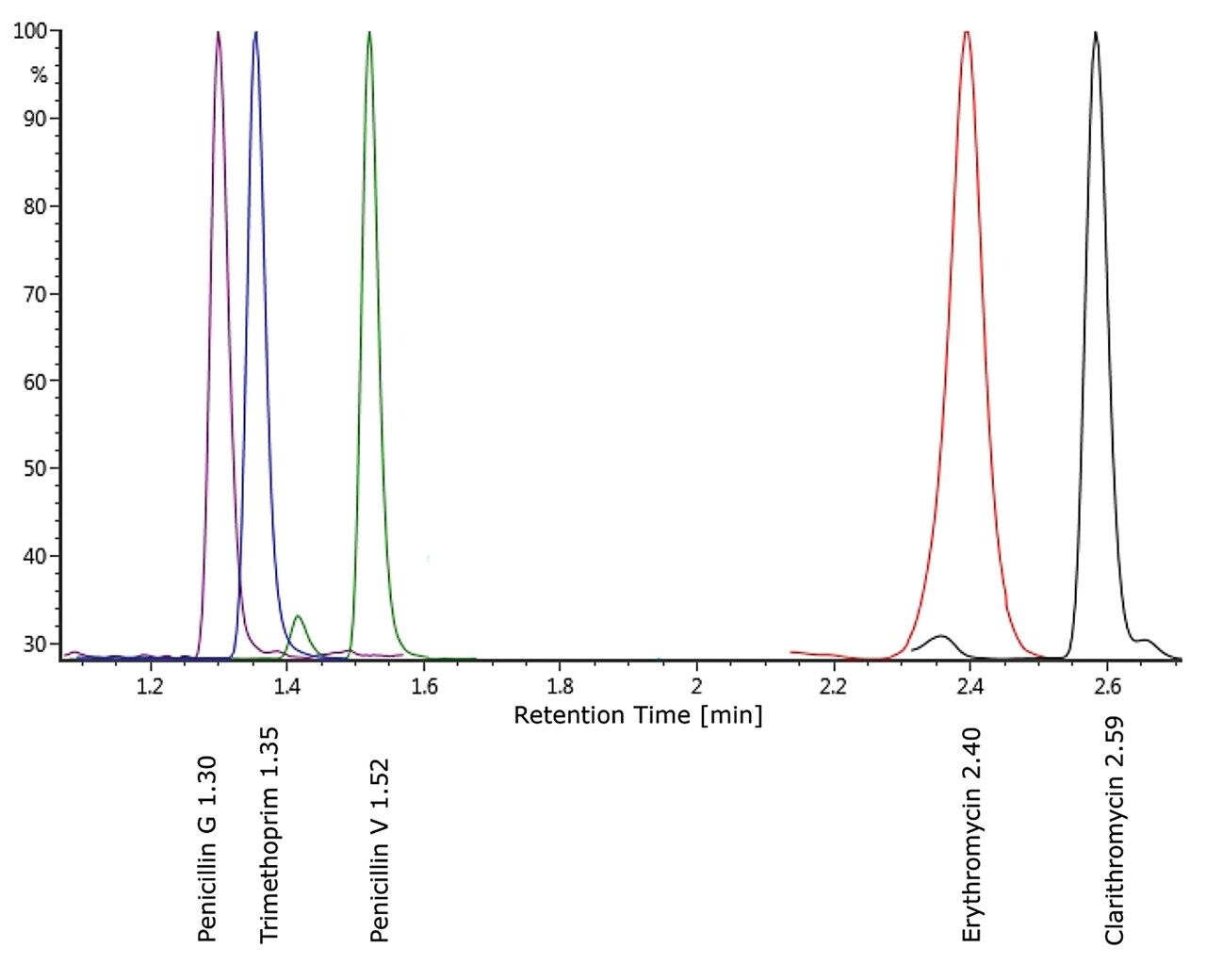
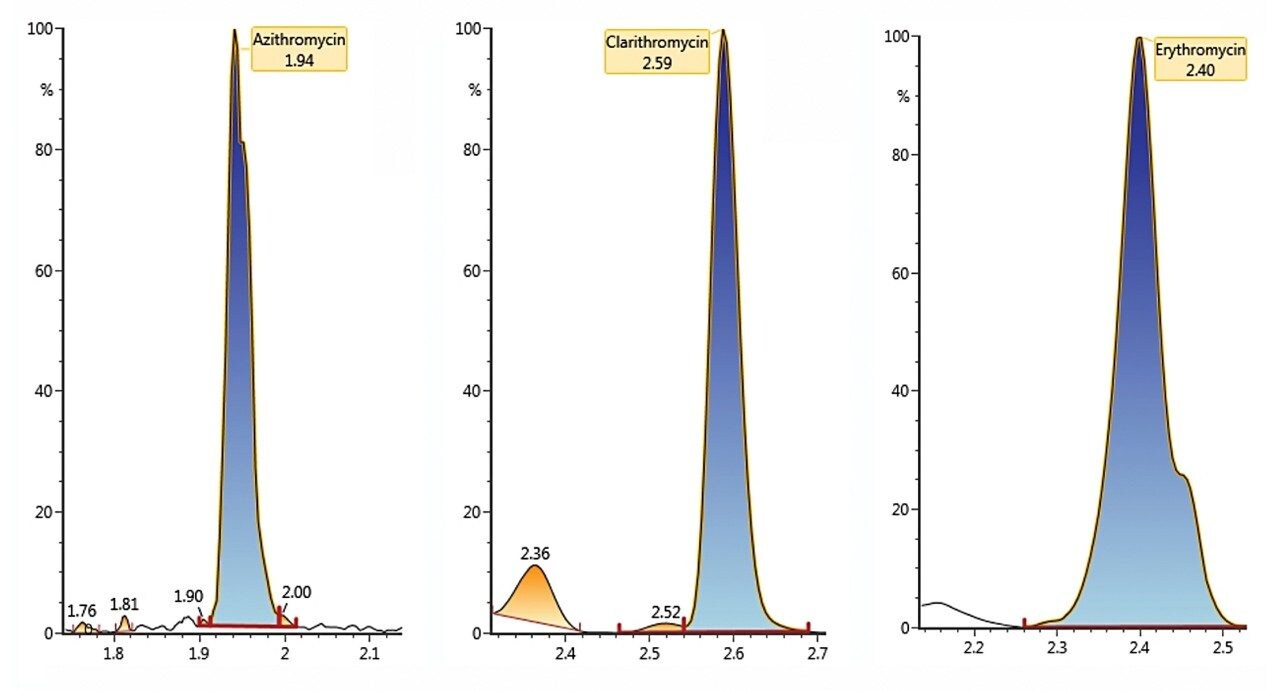
Example chromatography for antibiotics, fortified at approximately 100 ng/L in humic rich surface water using the basic conditions chromatography method, is shown in Figure 3. Chromatograms showing the detection of azithromycin, clarithromycin, and erythromycin in WWTP effluent samples from the City of Kristianstad Sweden is shown in Figure 4. Analysis of the WWTP effluent water, as well as a surface water from Lake Hammarsjön, which is downstream of the WWTP, revealed the presence of all of the macrolides on the EU watch list in the range of 2.6 to 215 ng/L. Penicillin G and V were not detected, most likely since they belong to the ß-lactams which are easily degraded. Ciprofloxacin and norfloxacin were also not detected, as these have been shown to bind to the solid sludge phase in the WWTPs. The presented methodology is flexible and allows for a simple introduction of new antibiotics with minimal time spent on method development which is demanding and costly.²
720006003, May 2013