This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates an optimized method for the routine analysis of tobacco specific nitrosamines (TSNAs) in tobacco products using RADAR Technology.
RADAR enables simultaneous acquisition of both MRM and full scan MS data to simplify and accelerate the development of robust methods.
Tobacco specific nitrosamines (TSNAs) are a group of carcinogenic compounds found in tobacco and tobacco smoke. Several priority toxicants including TSNAs have been identified in tobacco and smoke emissions that need to be accurately measured and reported to regulatory bodies. Four different TSNAs are monitored in tobacco product analysis: N-nitrosonornicotine (NNN), 4-(N-methylnitrosamino 1-(3-pyridyl)-1- butanone (NNK), N-nitrosoanatabine (NAT), and N-nitrosoanabasine (NAB).
Tobacco and smokeless tobacco products such as snus, moist snuff, dry snuff, and chewing tobacco were studied. As tobacco products contain high amounts of nicotine and other alkaloids, it is difficult to develop sample preparation procedures to selectively remove these interferences for TSNA analysis. These co-eluting interferences can compete with the analyte of interest during the ionization process, which can lead to suppression or enhancement of the analyte signal. Hence, the elution of analytes by minimizing matrix effects via SPE cleanup or sample dilution is critical for a robust and rugged LC-MS/MS method for analysis of nitrosamines.
Waters Xevo TQD Triple Quadrupole Mass Spectrometry System employing RADAR functionality provides an innovative platform to monitor both MRM and full scan MS data simultaneously without compromising data quality. The full scan data helps characterize the potential matrix interferences and enables informed decision making during the method development process. In this study, an optimized UPLC-MS/MS method for TSNA analysis in tobacco products has been developed using RADAR Technology.
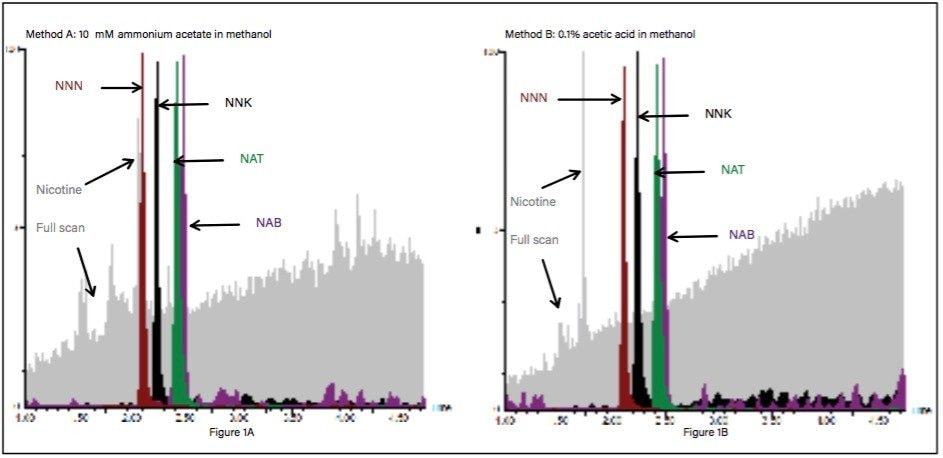
To optimize the chromatographic separation for TSNAs, RADAR acquisition mode was utilized to characterize the tobacco background matrix. RADAR enables simultaneous acquisition of full scan MS and MRM data, a unique capability that can both simplify and accelerate development of robust methods. Two different organic based mobile phase modifiers (mobile phase B) were evaluated using the same gradient profile: 10 mM ammonium acetate in methanol (Method A) and 0.1% acetic acid in methanol (Method B). The same mobile phase A (10 mM ammonium acetate in water) was used for both the methods. Figure 1A shows an overlay chromatogram of all four nitrosamine MRMs with simultaneously acquired full scan data using Method A. As can be seen in Figure 1A, the NNN peak is co-eluting with the high intensity nicotine peak. Figure 1B shows an overlay chromatogram of all nitrosamine MRMs with full scan data using Method B. Nicotine (1.75 min) and NNN (2.15 min) peaks were well separated using the acidic mobile phase. Nicotine is dibasic in nature, thus the protonated and unprotonated (free nicotine) forms are pH dependent. At low mobile phase pH, the acidic modifier facilitates the separation of nicotine and NNN, as shown in Figure 1B.
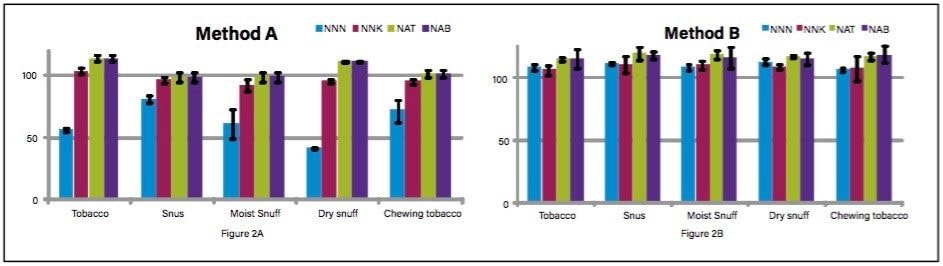
To assess method accuracy, nitrosamines were spiked at 50 ng/g of NNN, NNK, NAT, and 12.5 ng/g of NAB in triplicates, then acquired using Methods A and B, and quantified against solvent calibration curves. For both methods, two internal standards (NNN-D4 and NNK- D4) were employed to calculate accurate quantification. Figure 2A and 2B shows quantification results (method recoveries) for all nitrosamines in various tobacco products using Methods A and B. As shown in Figure 2A, NNN quantification results (method recoveries) from various tobacco products were below the 80% to 120% acceptance criteria. Due to the co-eluting nicotine peak, significant matrix suppression was observed for NNN in Method A resulting in lower quantification. Method B showed improvement for NNN recoveries in all tobacco products over Method A, and the observed concentration ranged from 106% to 112% with standard deviation less than 2.7% using Method B.
This technology brief demonstrates the utility of the RADAR functionality to optimize TSNA analysis in tobacco products. RADAR enables simultaneous acquisition of both MRM and full scan MS data, a unique capability that can both simplify and accelerate development of robust methods. The ability to review qualitative and quantitative data acquired simultaneously enables development of analytical strategies to overcome matrix effects and to improve data quality.
720005795, October 2016