
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates to replace titrimetric and flow injection analysis of 1-(3-dimethylaminopropyl)- 3-ethylcarbodiimide hydrochloride raw material with an alternative approach using an ACQUITY UPLC System with an ACQUITY QDa Detector.
The ACQUITY UPLC System coupled with an ACQUITY QDa Detector eliminates titrimetric and flow injection techniques and provides simplistic analysis of the EDC·HCl raw material.
Pharmaceutical raw materials are substrates or elements used for manufacturing different drug products. Raw materials include active pharmaceutical ingredients (API), excipients, and other inactive ingredients. Excipients and inactive ingredients generally have no pharmacological effect, yet they are essential components that function as fillers, binders, disintegrants, lubricants, coloring agents, and preservatives.1
1-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride (EDC•HCl) is a cross linking reagent used for peptide synthesis, protein crosslinking to nucleic acids, and in preparation of immunoconjugates. Methods for analysis of EDC•HCl found in the literature include a fluorometric method coupled with a titration technique2 and spectrophotometric flow injection analysis.3 While effective, these methods are not ideal for QC laboratories. The titration technique lacks reproducibility required for routine testing, while the flow injection analysis depends on reaction efficiency and often lacks the sensitivity required for analysis of pharmaceutical samples.
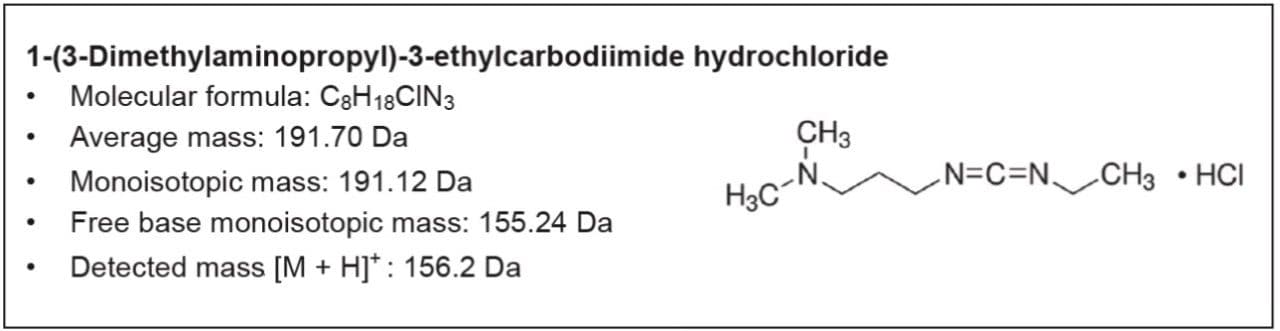
Here we describe a robust and quick UPLC method for analysis of EDC•HCl. This methodology was developed in partnership with a large multinational biopharmaceutical company. The UPLC method utilizes an ACQUITY QDa Detector for fast, information-rich, and accurate testing of raw materials.
The sample used in this study was prepared by dissolving EDC•HCl material in water to make a stock solution at 0.5 mg/mL. The stock solution was then diluted with water to a working concentration of 2.0 μg/mL.
The chromatographic separation was performed on an ACQUITY UPLC H-Class System, using the method conditions described in Figure 2.
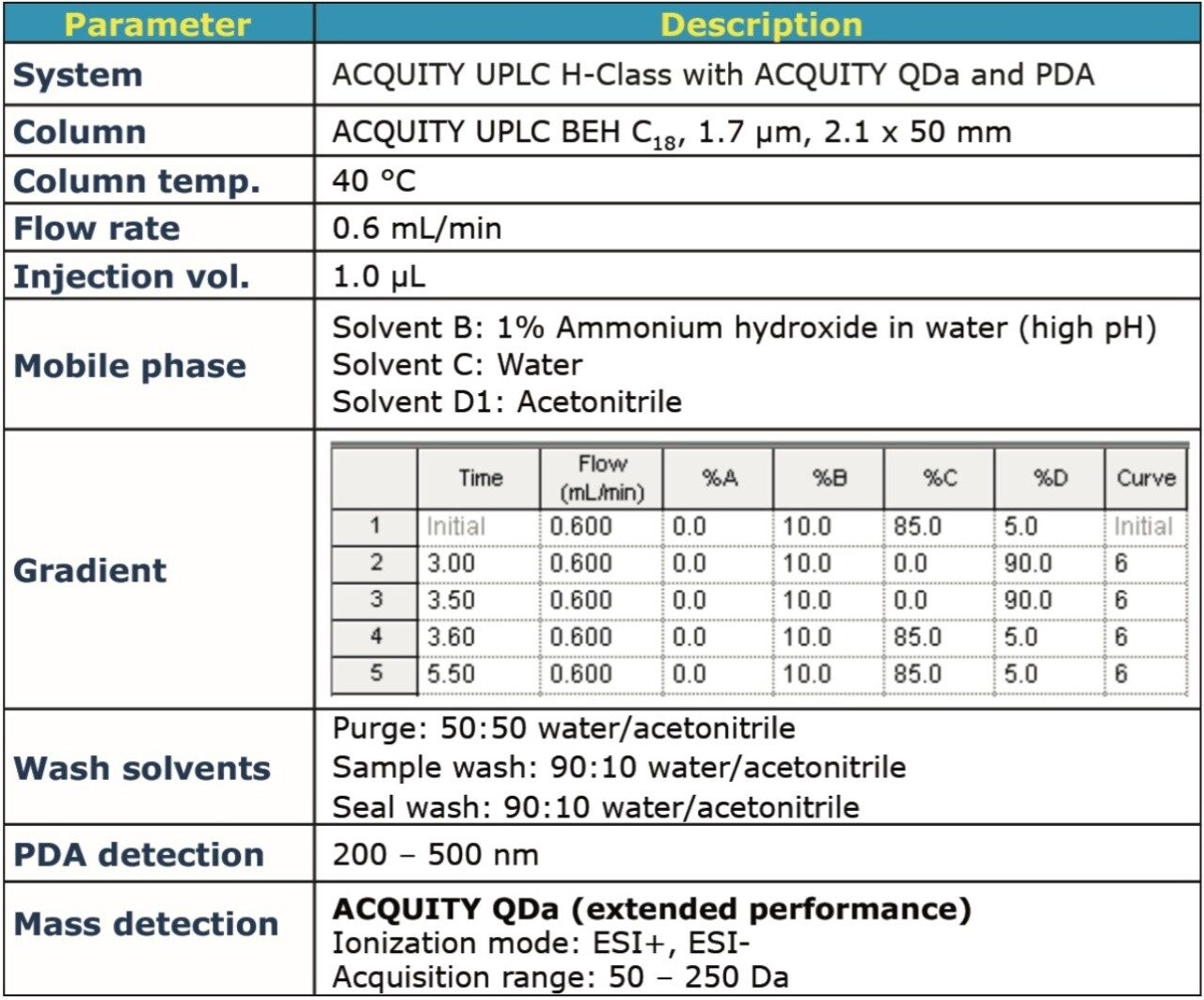
The chromatographic data for analysis of EDC•HCl is displayed in Figure 3. The UV trace at 215 nm (Figure 3A) shows that a small peak was detected, indicating that EDC•HCl exhibits poor UV absorbance. The ACQUITY QDa Detector data collected across the entire mass range (50 – 250 Da, Figure 3B) is referred to as the total ion chromatogram (TIC). A specific mass of interest can be extracted from the scanning data (TIC) to generate an extracted ion chromatogram (XIC) as illustrated in Figure 3C. In this case we monitor the mass-to-ratio (m/z) of 156.2 Da corresponding to EDC•HCl. For targeted assay analyses, the data can be collected using single ion recording (SIR) mode, which records the signal intensity for a specific ion of interest and can simplify both the analysis and increase of S/N further, Figure 3D.

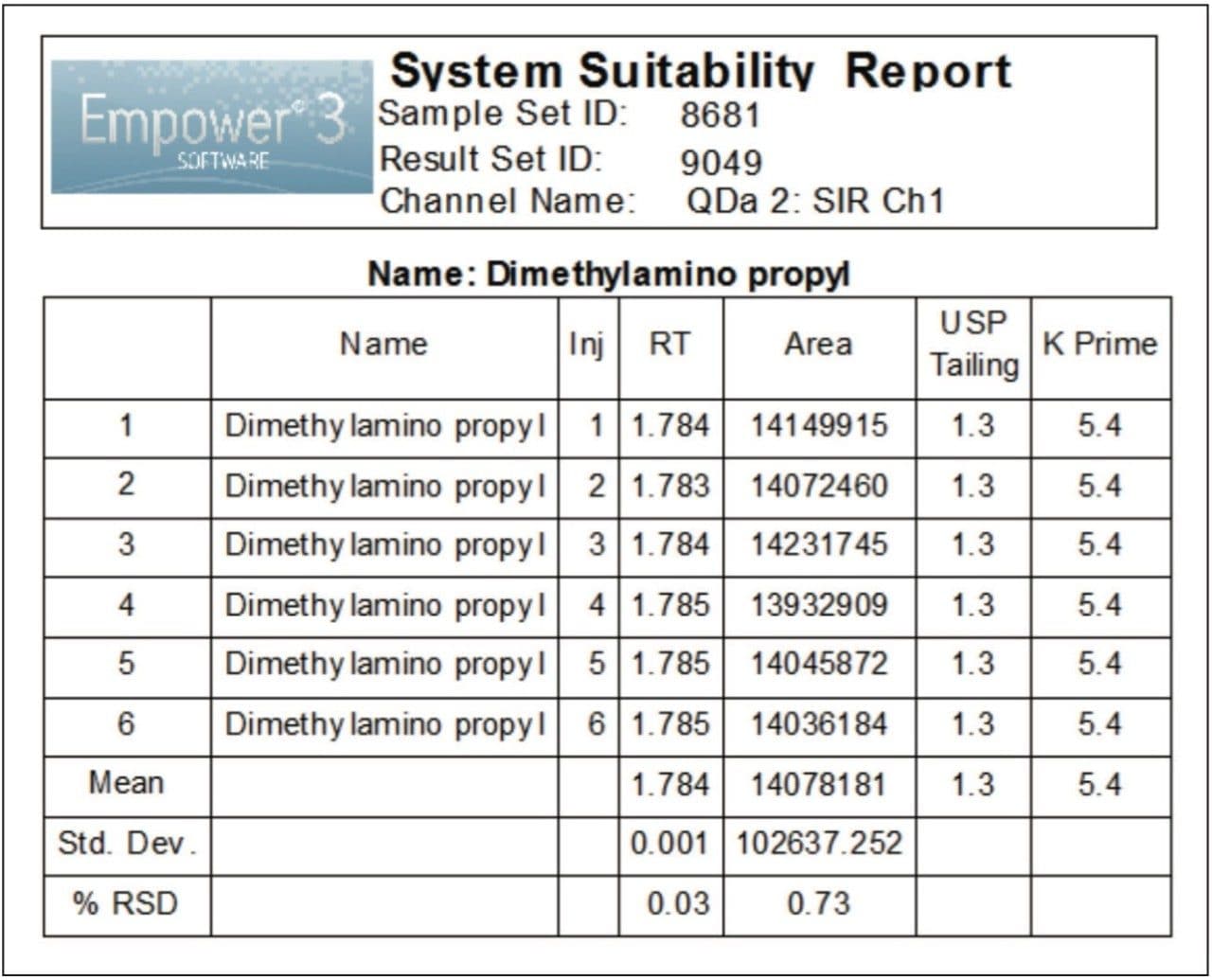
The performance of the UPLC method was verified by evaluating repeatability of six replicate injections of the 2.0 μg/mL standard according to the specifications defined in the USP General Chapter, <621>, Chromatography.4 The ACQUITY UPLC System suitability results, processed using SIR collected for a mass of 156.2 Da, are shown in Figure 4. The retention times and area repeatability were well within the USP specifications of less than 2% relative standard deviation (RSD).
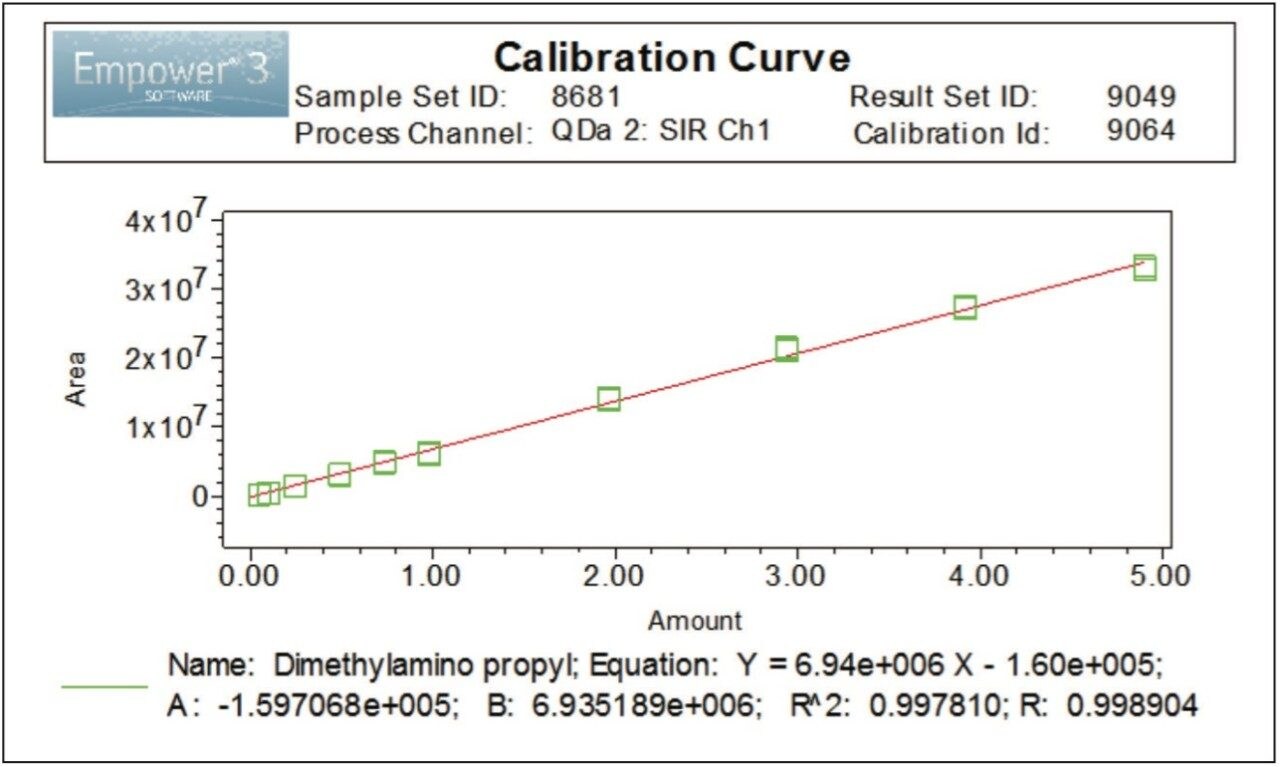
Linearity of the method for EDC•HCl with SIR at 156.2 Da was evaluated over 10 concentration levels ranging from 0.05 to 5.00 μg/mL. The method showed good linear behavior with a correlation coefficient (R2) ≥0.997, Figure 5.
The ACQUITY UPLC System coupled with an ACQUITY QDa Detector eliminates the titrimetric and flow injection techniques and provides simplistic analysis of the EDC•HCl raw material. System suitability and linearity of the method calculated using mass data were excellent. The new method provides improved confidence associated with sample confirmation and increased productivity.
Overall, the ACQUITY QDa Detector is a robust and simple-to-use mass detector that can be added as an orthogonal detection technique to UV detection. It provides accurate and reliable results, making this technology ideal for routine testing in the QC laboratory.
720005294, February 2015