This study illustrates the rapid and efficient separation of all six of the possible positional isomers of both the six disubstituted dimethoxybenzoic acids and also the six dimethylbenzoic acids.
Positional isomers are compounds that only differ in the location of the substituent groups. Each one of these isomers differs only slightly in physical properties from the others. For this reason, positional isomer mixtures can often pose serious challenges in attempts to achieve their separations. This study illustrates the rapid and efficient separation of all six of the possible positional isomers of both the six disubstituted dimethoxybenzoic acids and also the six dimethylbenzoic acids (DMBA) (Figure 1). This latter example is particularly interesting, as DMBA is a metabolite of trimethylbenzene (TMB) and its appearance in human urine can be used as a clinical marker for measuring industrial exposure to TMB.1
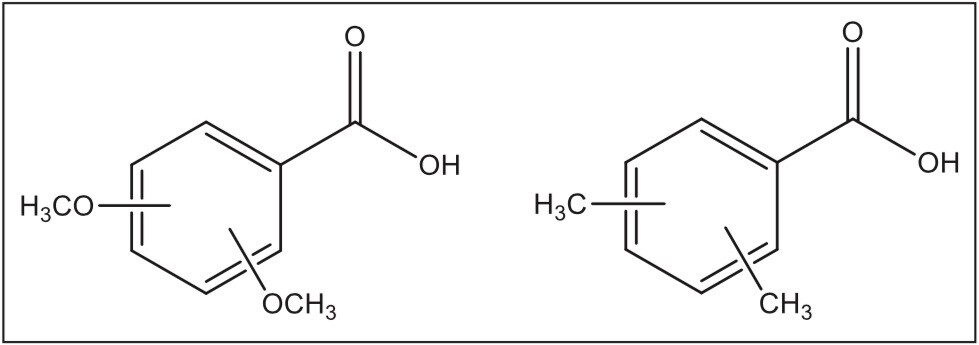
The six isomer standards for the disubstituted dimethoxybenzoic acids and the dimethylbenzoic acids were obtained from Sigma-Aldrich and from TCI Americas respectively. Samples were dissolved in methanol at a concentration of 0.1 mg/mL.
|
UPC2 conditions |
|
|---|---|
|
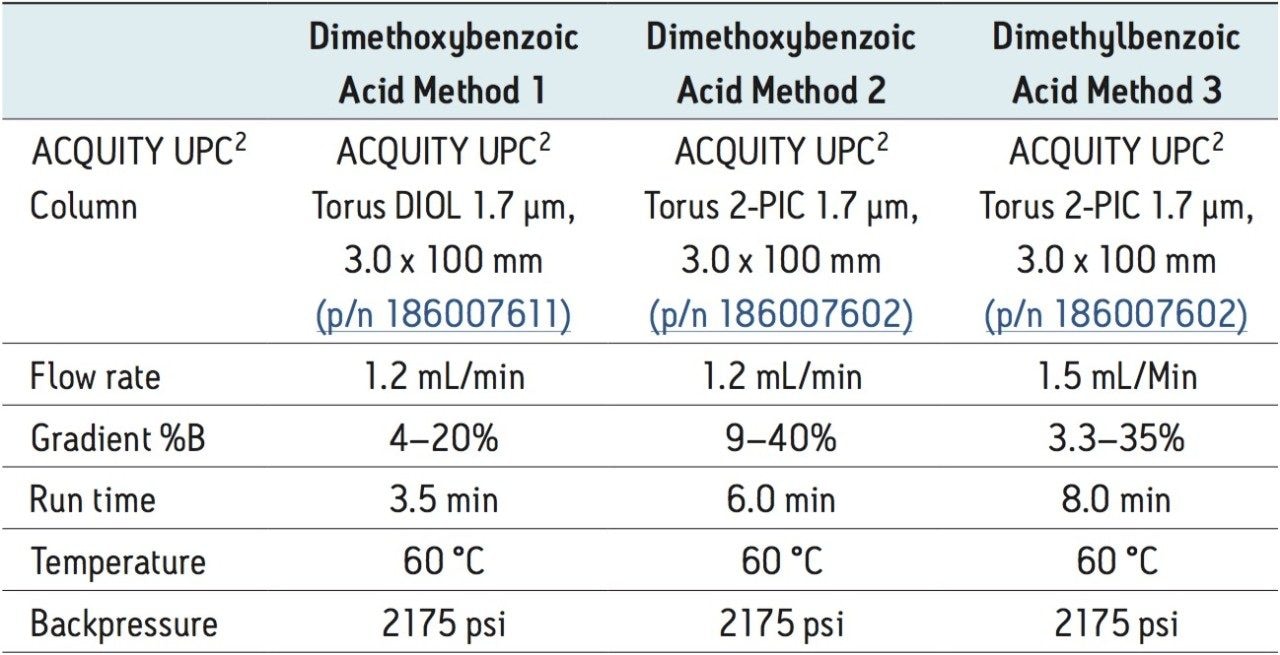
See Table 1 |
|
|
Mobile phase A: |
Supercritical CO2 |
|
Mobile phase B: |
Methanol containing 0.2% formic acid |
|
Vials: |
12 x 32 mm glass screw neck vials with Quick Thread Lectra Bond caps with preslit PTFE/Silicone septa (p/n 186000307C) |
|
PDA detector: |
240 nm wavelength compensated (340–410 nm) |
|
System: |
Waters TQD |
|
|
Ionization mode: |
ESI- |
|
|
Acquisition range: |
150–650 amu |
|
|
Capillary voltage: |
3.58 kV |
|
|
Cone voltage: |
20 V, SIR:DMBA 149.1 amu, dwell 0.1 |
|
|
Chromatography and MS Software: |
MassLynx |
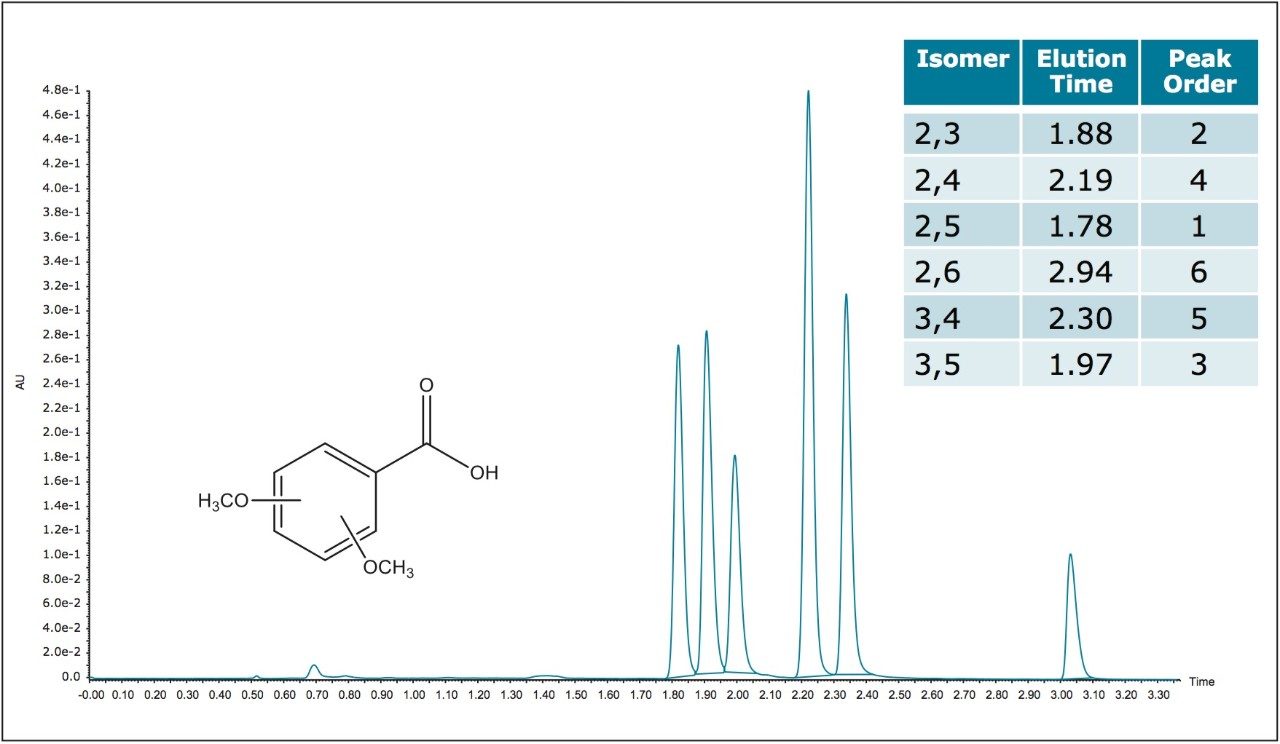
Due to the very similar physical properties of positional isomers, their separations have often proven to be quite challenging. Substitution patterns, such as groups located in the ortho, meta, and para positions of the aromatic ring do have effects on the electronic contributions to aromatic resonance. Ortho substitutions also contribute to the level of the steric hindrance in the carboxylic acid group environment, which affects its ability to maintain coplanarity with the aromatic ring. All of these subtle differences contribute to small differences in pi cloud effects in the ring and to the pKa of the acid itself. These small steric and electronic differences are also manifested in the different extinction coefficients for each of the positional isomers. Such subtle property differences are also the basis upon which separation of the individual positional isomers is possible. Isomer separations are often required for starting material qualifications, reaction monitoring and in an example which will be shown here, for following a particular isomer which is a known metabolite resulting from exposure to a toxic material and which is used as a clinical marker for industrial exposure.1 The ACQUITY UPC2 System utilizing ACQUITY UPC2 Torus Column chemistries is capable of such separations, as several examples presented here can demonstrate. The Torus Column family is a new generation of stationary phases designed specifically for UPC2 separations. Torus Technology is built upon proven BEH (ethylene bridged hybrid) particles as a proprietary two stage bonding process. The first stage of the bonding controls the retention characteristics of the phase and the second imparts the observed selectivity. In the first example shown separation of six isomers of dimethoxybenzoic acids is accomplished using the ACQUITY UPC2 Torus DIOL Column (Figure 2). Separations with a diol phase are based on polar interactions and hydrogen bonding interactions. This same set of isomers can also be separated with the ACQUITY UPC2 Torus 2-PIC Column chemistry (Figure 3).
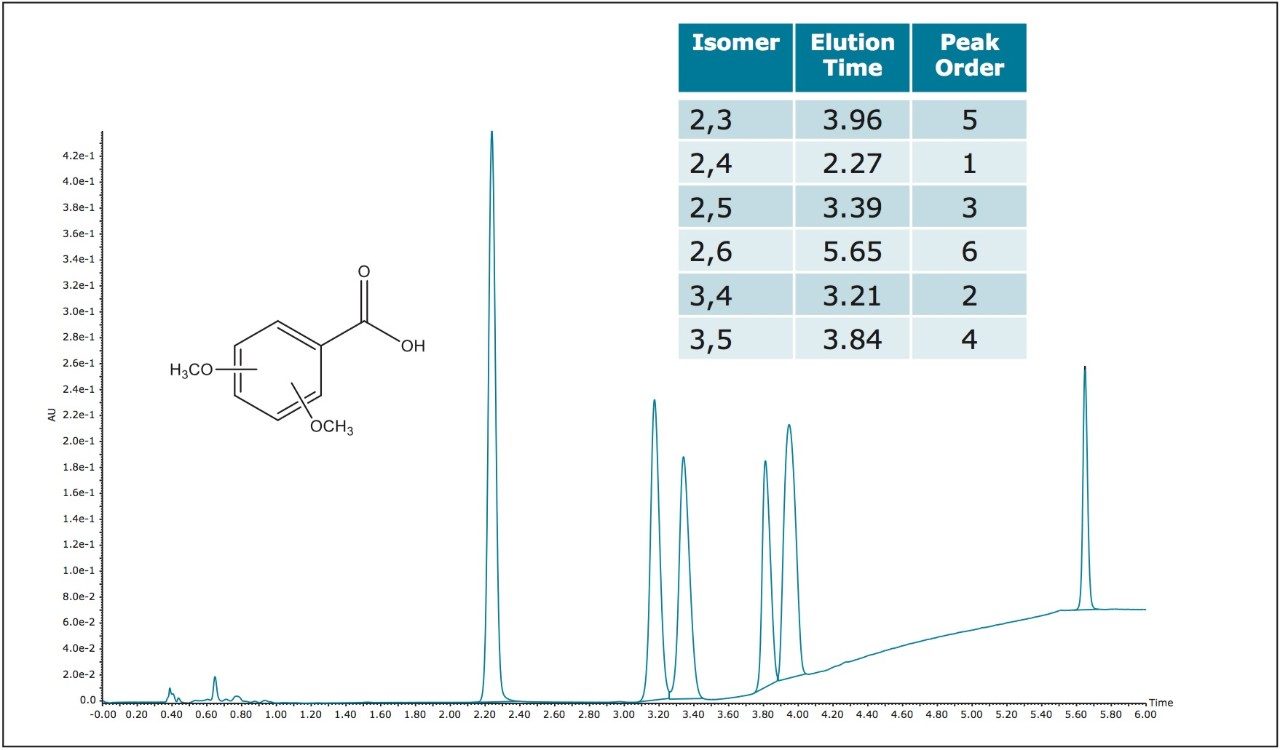
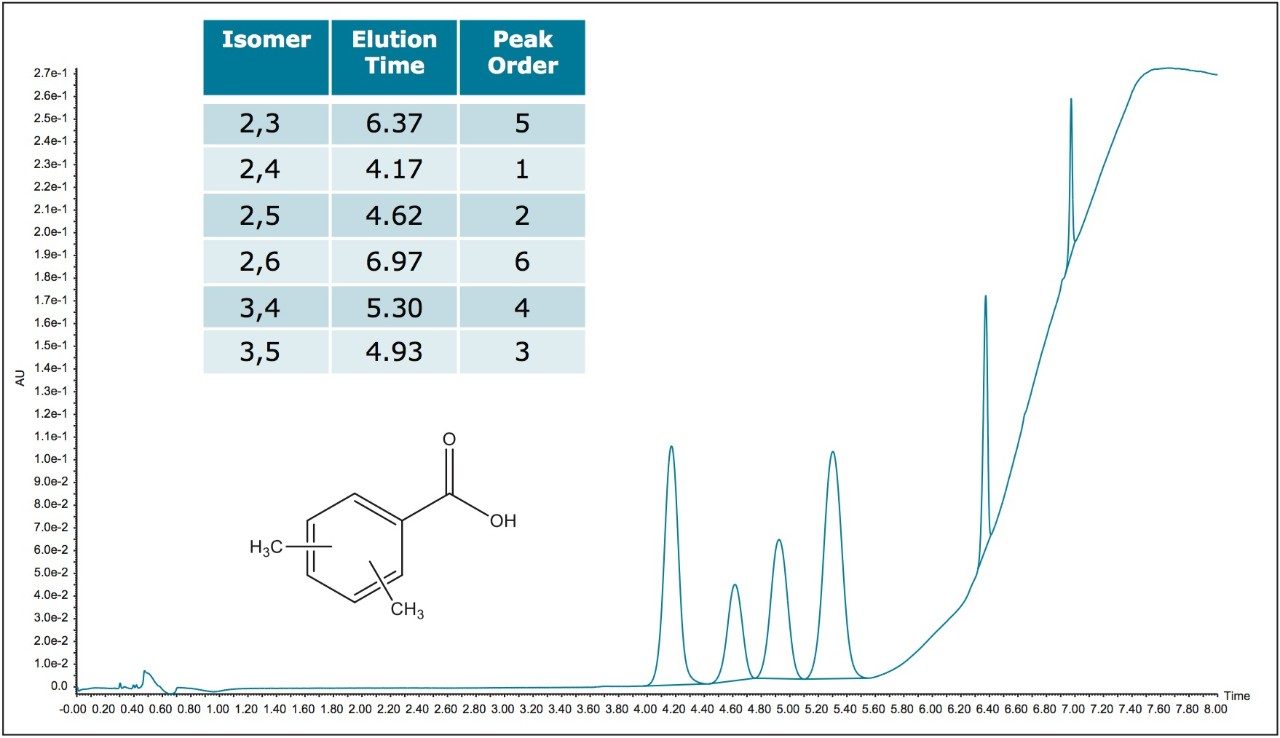
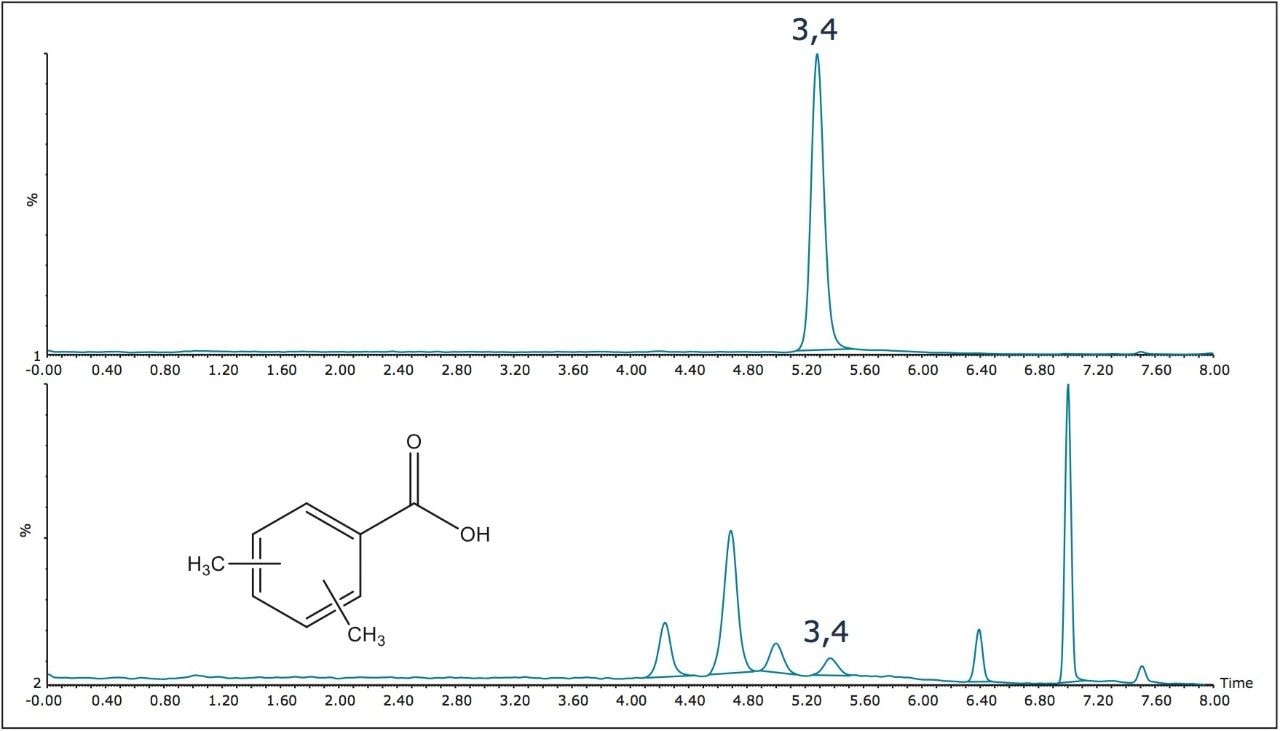
The ACQUITY UPC2 2-PIC phase also has the possibility of separating with acid-base and pi-pi interactions, and in fact the isomer elution orders are different on the two different phases. The ACQUITY UPC2 Torus 2-PIC Column is also capable of separating all six of the positional isomers of the dimethylbenzoic acids (DMBA) which as mentioned before, has been used as a metabolic clinical marker (Figure 4). The 3,4 dimethyl isomer, peak 4 in Figure 4, which is of the greatest clinical interest is well separated from the other isomers. The chromatography figures shown are all wavelength compensated UV traces, but it is also possible to monitor these peaks using negative ion mass spectrometry. While all the peaks are isobaric, MS monitoring does allow for gains in sensitivity and specificity, which is especially important in clinical metabolite monitoring. In fact, using SIR (selective ion recording) methods it is possible to reach much higher sensitivities which are well below the LOQ for UV monitoring, while at the same time reducing interferences from many matrix effects. The SIR recording of a chromatogram from a more dilute DMBA sample mix, using the same chromatographic method as before, is shown over layed with a standard for the 3,4 DMBA isomer alone (Figure 5). Actual clinical applications are beyond the scope of this study, although it is clearly shown that it is possible to employ ACQUITY UPC2 methods, working with the ACQUITY UPC2 Torus Columns, using techniques which are derivatization free, to monitor these positional isomers using SIR MS detection. This is compared to the current GC methods, which do require a silylation derivative forming reaction step, the efficiency of which is another possible source for error.1
The positional isomers of two examples of disubstituted benzoic acids are rapidly and efficiently separated into their individual components with ACQUITY UPC2 Torus Column chemistries using the ACQUITY UPC2 System. This separation is accomplished without any need for prior derivatization reactions, as is necessary in the competing GC methods.1 In one example, for DMBA on the ACQUITY UPC2 Torus 2-PIC Column, this separation is demonstrated to be highly sensitive and selective with the use of MS SIR monitoring detection techniques.
720005221, November 2014