This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrate the unique ability of charged surface hybrid (CSH Technology) C18 to produce high peak capacity peptide separations at high mass loads in nanoLC chromatography
CSH130 C18 capillary columns offer unique selectivity and produce remarkable peak capacities at high mass loads.
LC-MS techniques once relegated to shotgun proteomics are now being exploited to elucidate cellular mechanisms and to discover as well as monitor biomarkers. For instance, phosphopeptide analysis by nanoLC-MS has become a standard approach in drug discovery, wherein comparative data are mined to identify therapeutic targets and better understand drug mechanisms of action. Capillary (i.e. 75 μm ID) columns and nanoliter per minute flow rates are often employed in these applications in order to derive an abundance of information from a relatively minimal amount of sample.
Obtaining high peak capacity peptide separations at high mass loads is a highly desirable attribute for these examples of capillary chromatography, since this would increase the ability to detect trace species without compromise in terms of co-elution. For analytical scale chromatography, a novel, charge surface modified C18 stationary phase, CSH130 C18, has been used with good effect to produce such separations. The positive surface potential exhibited by CSH130 C18 at an acidic pH significantly enhances its loadability by minimizing undesired secondary interactions involving peptide analytes. Here, it is shown that in application to nanoLC capillary chromatography, the extreme loadability of CSH130 C18 makes it possible to load inordinately high quantities of protein digest onto a small ID column without detriment to chromatographic performance. This characteristic of CSH130 C18, in addition to its unique selectivity, makes it a useful alternative to more conventional C18 stationary phases, including hybrid organo-silica (BEH130 and BEH300 C18) and 100% silica-based (HSS T3) stationary phases without modified surface potentials.
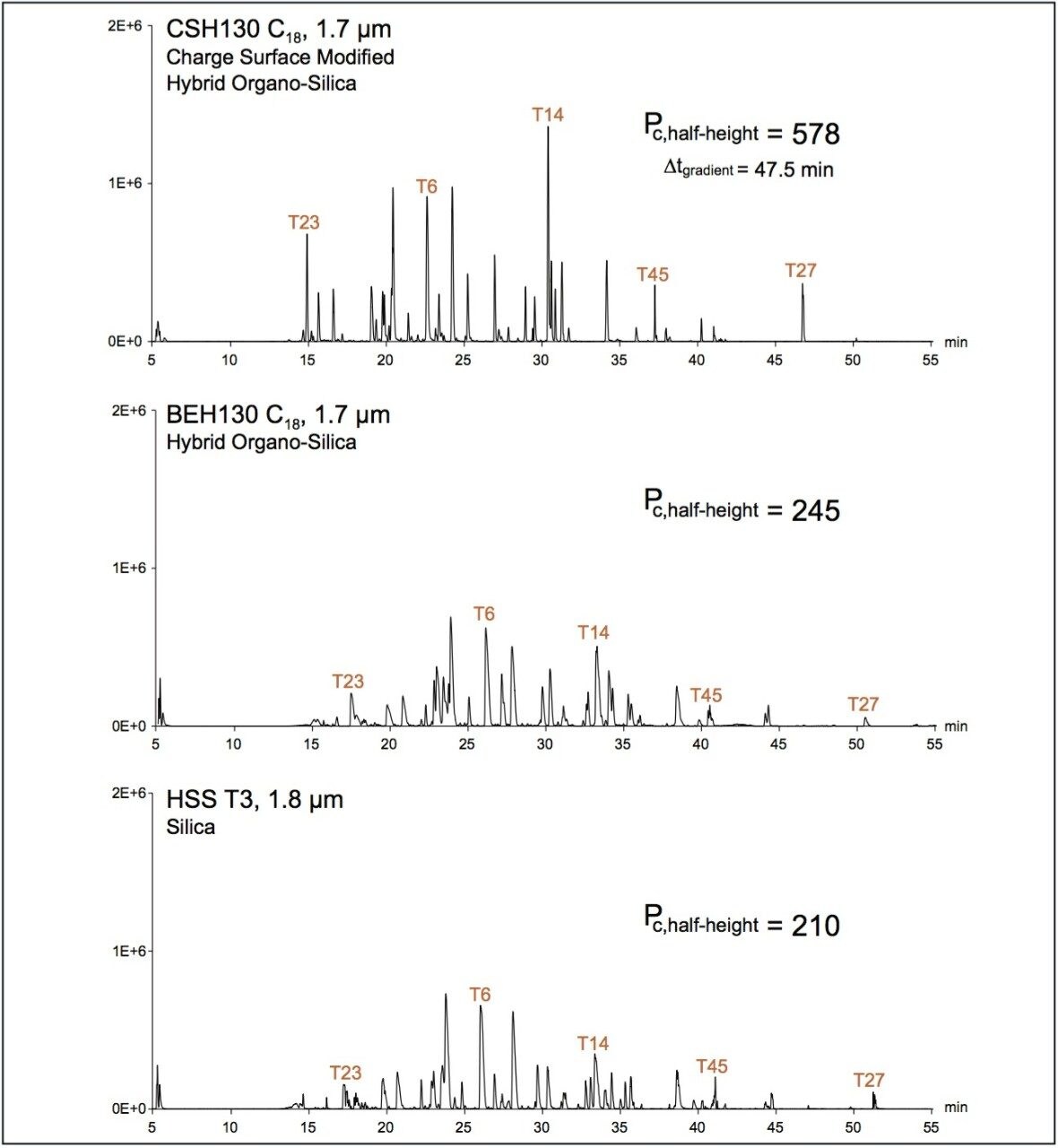
The performance of three chemically unique C18 stationary phases was investigated via application to nanoLC peptide separations. Specifically, a large quantity of trypsin-digested yeast enolase (ca. 1 μg of a ~50 kDa protein) was analyzed by LC-ESI/MS using a 75 μm ID capillary column packed with either sub-2-μm charge surface modified C18 (CSH130 C18), organo-silica hybrid C18 (BEH130 C18), or 100% silica-based C18 (HSS T3). Figure 1 shows the base peak intensity chromatograms (BPIs) obtained with each of these materials and 0.1% formic acid mobile phases. The most striking differences among these results reside in comparison of the separation obtained with the charge surface modified C18 versus those obtained with the other column chemistries. Clearly, peak shape is improved with the CSH130 C18 material, a phenomenon that has now been well documented for peptide separations at an analytical scale (Anal. Chem., 2013, 85 [14], pp 6936–6944). Peak capacities based on extracted ion chromatograms of five enolase tryptic peptides (Enolase T23, T6, T14, T45 and T27) corroborate this assessment (Figure 1). Where the BEH130 C18 and HSS T3 phases produced peak capacities of 210 and 245, respectively, CSH130 C18 yielded a peak capacity of 578.
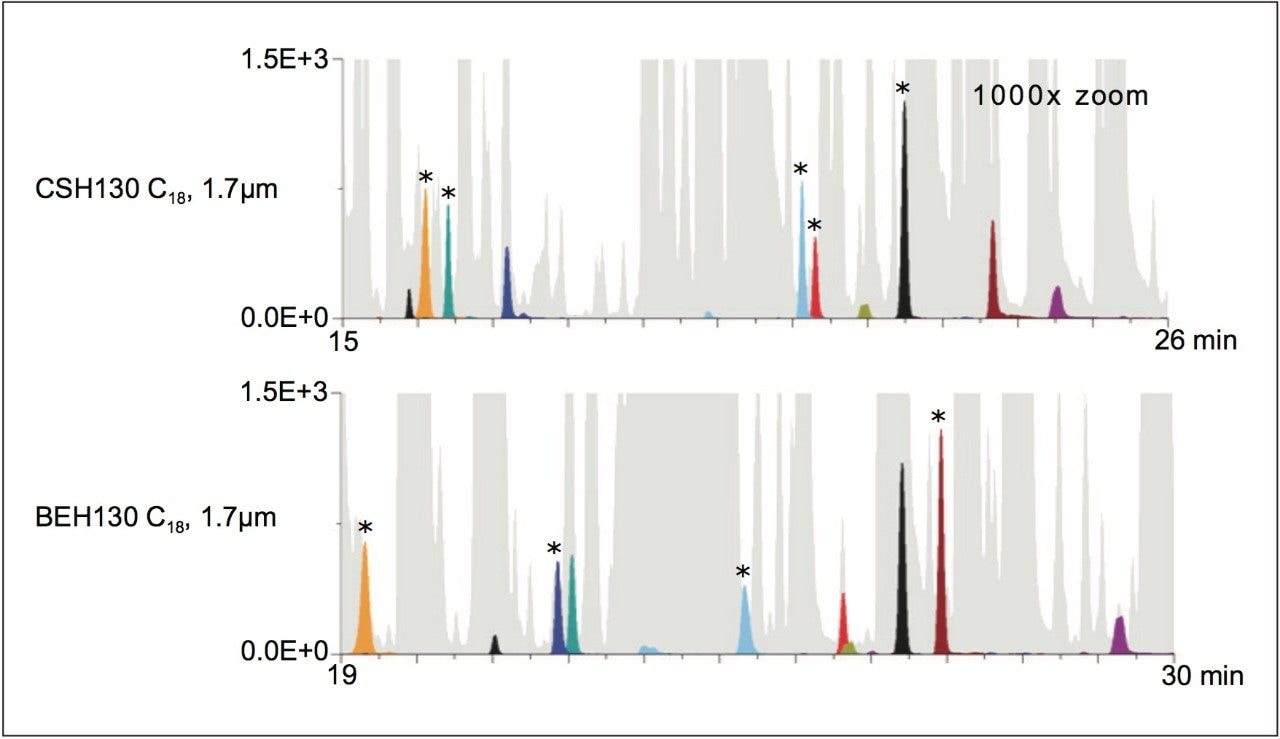
Obtaining high peak capacities at high mass loads improves the ability to detect low abundance species and reduces co-elution among components of a complex sample. For fragmentation analysis, this typically corresponds to improvements in spectral quality, given that ion suppression and spectral crowding is minimized. Displayed in Figure 2 is a set of windows showing a low intensity region of the CSH130 C18 and BEH130 C18 chromatograms. In Figure 2, the BPIs are shaded in gray. Since there are lesser amounts of gray in the CSH130 C18 figure (55 versus 61%), it can be said there is greater chance to detect low abundance species. To this point, close to 30 unique, low abundance peaks are observable in the low intensity region of the CSH130 C18 chromatogram, where as closer to only 20 unique peaks are observable in the BEH130 C18 chromatogram. Tracking nine different low abundance species in the enolase tryptic digest further demonstrates differences in these separations, including differences in co-elution. Using the CSH130 C18 column, five of the nine species were detected as the most abundant species in their respective ESI mass spectra (those marked with an asterisk). Meanwhile, four out of the nine low abundance species were detected as the most abundant species in their respective ESI mass spectra when using the BEH130 C18 column. The most intriguing observation from Figure 2, however, is that the co-elutions observed with the CSH130 C18 and BEH130 C18 columns appear to be largely distinct due to the significant differences in selectivity. These differences present the possibility of using a separation on each of these two C18 columns to increase the information that can be gleaned from a single sample.
Capillary columns, packed with CSH130 C18, are capable of producing remarkable peak capacities at high mass loads, such as those resulting from the analysis of ca. 1 μg of a single, trypsinized 50 kDa protein. As greater peak capacity and loadability are directly related to minimization of co-elution and the ability to detect low abundance species, CSH130 C18 holds significant promise for proteomics-related analyses. In particular, these attributes of CSH130 C18 are likely to make high quality fragment ion spectra more accessible for a greater number of peptides in a complex mixture. Moreover, CSH130 C18 exhibits unique selectivity compared to stationary phases without a positive surface potential, which suggests that use of both CSH130 C18 and BEH130 C18 columns can potentially produce unique identifications from a single sample, thereby providing the analyst with a more thorough characterization tool.
720004917, January 2014