
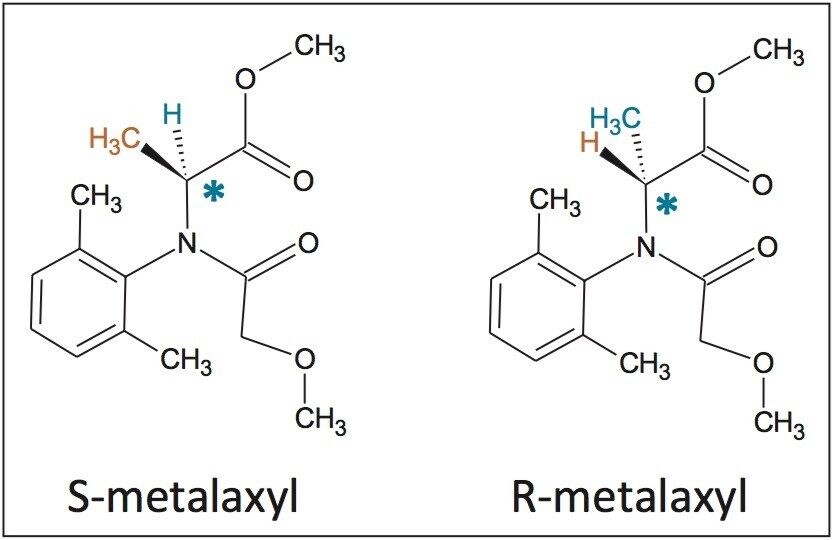
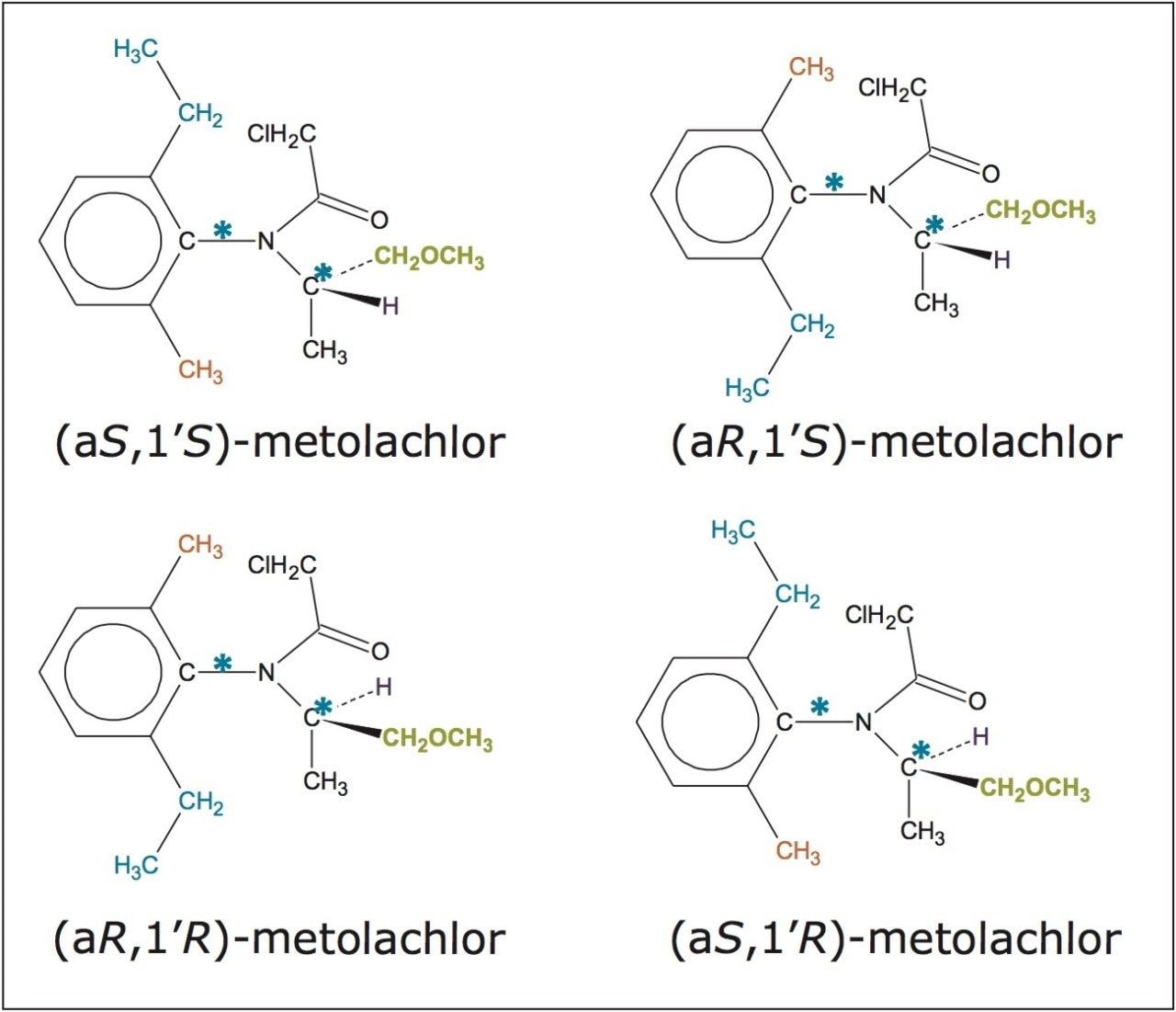
In this application note, the enantiomeric and/or diastereomeric resolutions of two pesticides, metalaxyl-M (a phenylamide fungicide) and S-metolachlor (acetanilide class of herbicides), and their analyses in aqueous-based pesticide formulations are presented.
Agricultural chemicals ensure that we have decreased crop damage resulting in a food supply that is plentiful and of high quality.1 In the agricultural chemicals manufacturing industry, the aim is to apply the least amount of pesticide that will give the most effective result. It is estimated that 30% of pesticides on the market today have optical isomers with reports that greater than 40% of the pesticides used in China are chiral.2,3 Biochemical reactions are usually stereo or enantioselective and, while one enantiomer may deliver the desired effect to the target species, the other enantiomer may be less effective to the target or completely ineffective. The presence of the less active enantiomer may not contribute to the efficacy of the formulation; however, it may have an enantioselective toxic effect on a non-target species. Chiral pesticides are often formulated as either racemic mixtures or a single biologically active isomer. Chiral separation is necessary for accurate measurement of the stereoisomers in the formulated product to ensure the correct active pesticide application rates.4,5 The study of chiral compounds has been a challenge due to the difficulty in resolving the stereoisomers. The most widely used analytical technique is separation using high-performance liquid chromatography (HPLC) with chiral stationary phases.3,6 Supercritical fluid chromatography (SFC) is known as an effective chiral separations technique, possessing many advantages over conventional HPLC.7,8 The properties of a supercritical fluid allow high efficiency separations with shorter analysis times. Convergence chromatography is a complimentary separation technique to liquid chromatography, providing orthogonal selectivity using supercritical CO2 as the primary mobile phase.
In this application note, the enantiomeric and/or diastereomeric resolutions of two pesticides, metalaxyl-M (a phenylamide fungicide) and S-metolachlor (acetanilide class of herbicides), and their analyses in aqueous-based pesticide formulations are presented. Metalaxyl (Figure 1) has one chiral center, and its biologically active form is the R enantiomer.9 Metolachlor (Figure 2) has two chiral centers and 95% of the herbicidal activity comes from the S enantiomers.10,11 Resolutions were performed using the Waters ACQUITY UPC2 System.
The pesticide formulations (Formulation 1 and Formulation 2) were weighed, and a volume of hexane, dichloromethane, and ethyl acetate were each tested. The resulting mixtures were sonicated, and the samples were syringe-filtered into an autosampler vial using a 0.2-μm PVDF filter, ready for sample analysis. Standard compounds were made up in solvents to match the sample diluents.
|
UPC2 conditions |
|
|
System: |
ACQUITY UPC2 with PDA detection |
|
Data management: |
Empower 3 Software |
|
Metalaxyl-M |
|
|
Separation mode: |
Gradient |
|
Column: |
CHIRALPAK IA-3 4.6 x 150 mm, 3 μm |
|
Co-solvent: |
2-propanol |
|
ABPR: |
2000 psi/138 bar |
|
Flow rate: |
4.0 mL/min |
|
UV detection wavelength: |
215 nm |
|
Column temp.: |
55 °C |
|
Injection volume: |
2 μL |
|
S-metolachlor |
|
|
Separation mode: |
Gradient |
|
Column: |
CHIRALPAK IA-3 4.6 x 150 mm, 3 μm |
|
Co-solvent: |
2-propanol |
|
ABPR: |
2000 psi/138 bar |
|
Flow rate: |
2.5 mL/min |
|
UV detection wavelength: |
220 nm |
|
Column temp.: |
35 °C |
|
Injection volume: |
2 μL |
The method development for the standard pesticides began with a generic screening gradient using a number of columns and co-solvents. The screening step can be completed rapidly due to the shorter analysis times that are possible using this technique. The combination of co-solvent and column that produced the most promising separation for each compound was then optimized. The selectivity in a chiral separation can change markedly by varying the temperature, pressure, and flow rate.12 The optimized resolutions for the standard materials of metalaxyl-M and S-metolachlor are shown in Figures 3 and 4.
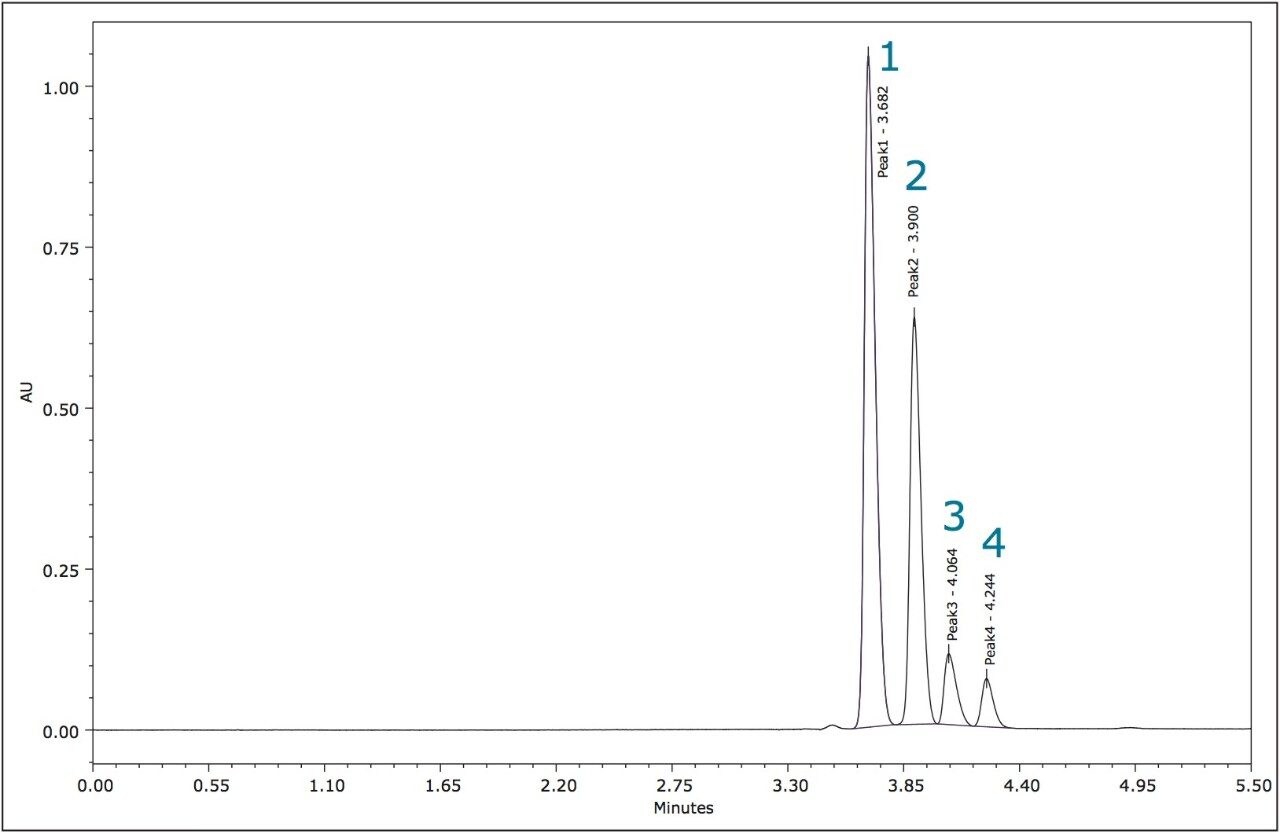
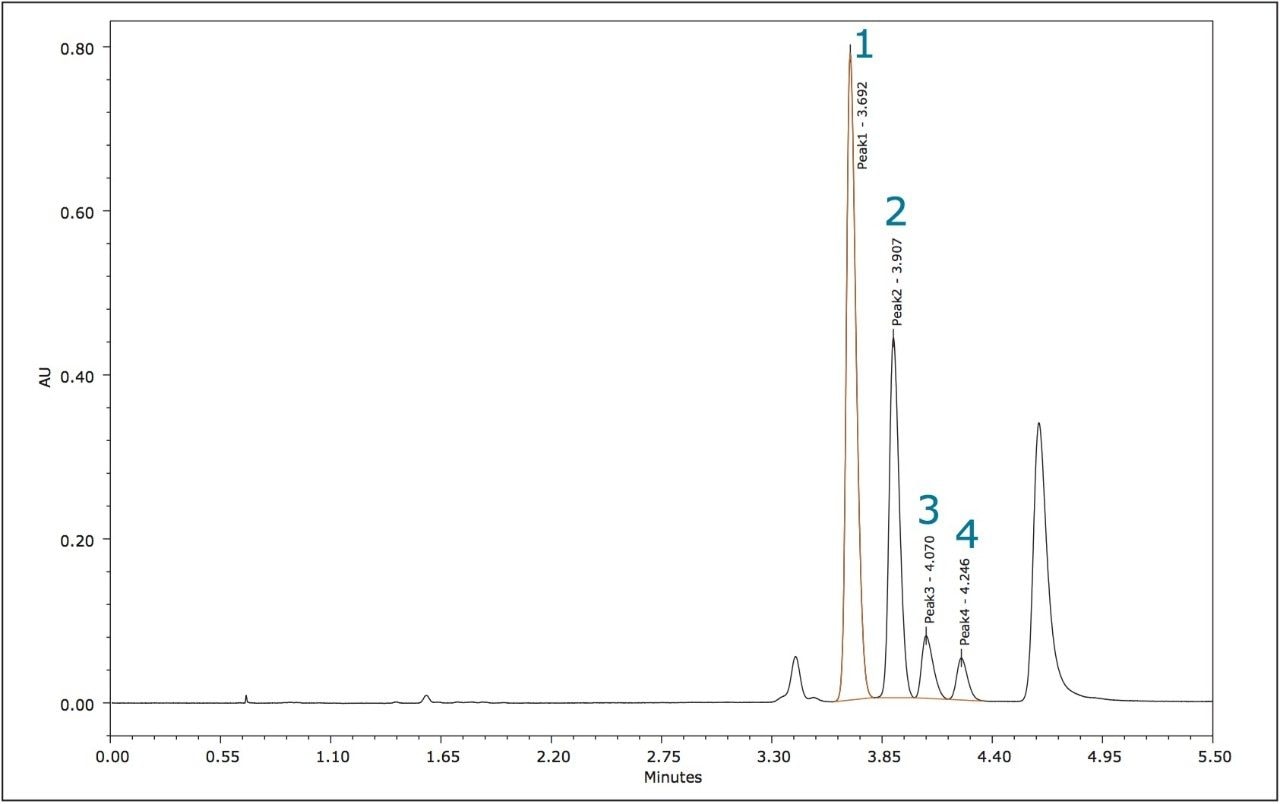
The relative peak areas in the chromatogram shown in Figure 4 indicate that the enantiomeric pairs for metolachlor are peaks 1,2 (S) and 3,4 (R).
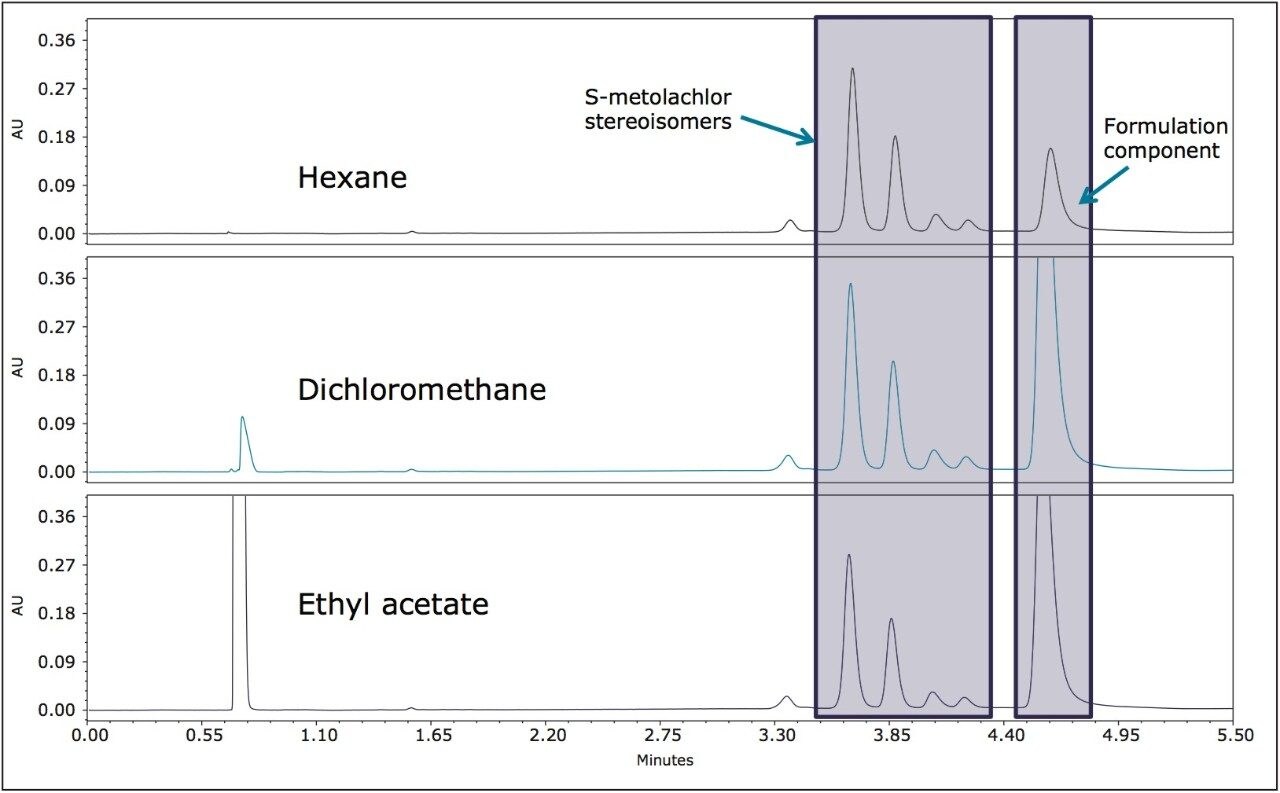
The pesticide formulations were aqueous-based and a solvent exchange was performed. Hexane, dichloromethane, and ethyl acetate were evaluated separately as extraction solvents. Hexane was more selective for less polar components in both formulations (Figures 5 and 6) and was used for further extractions. In this analysis, the goal was to resolve the stereoisomers and to reliably and reproducibly measure the ratios of the enantiomers/diastereomers.
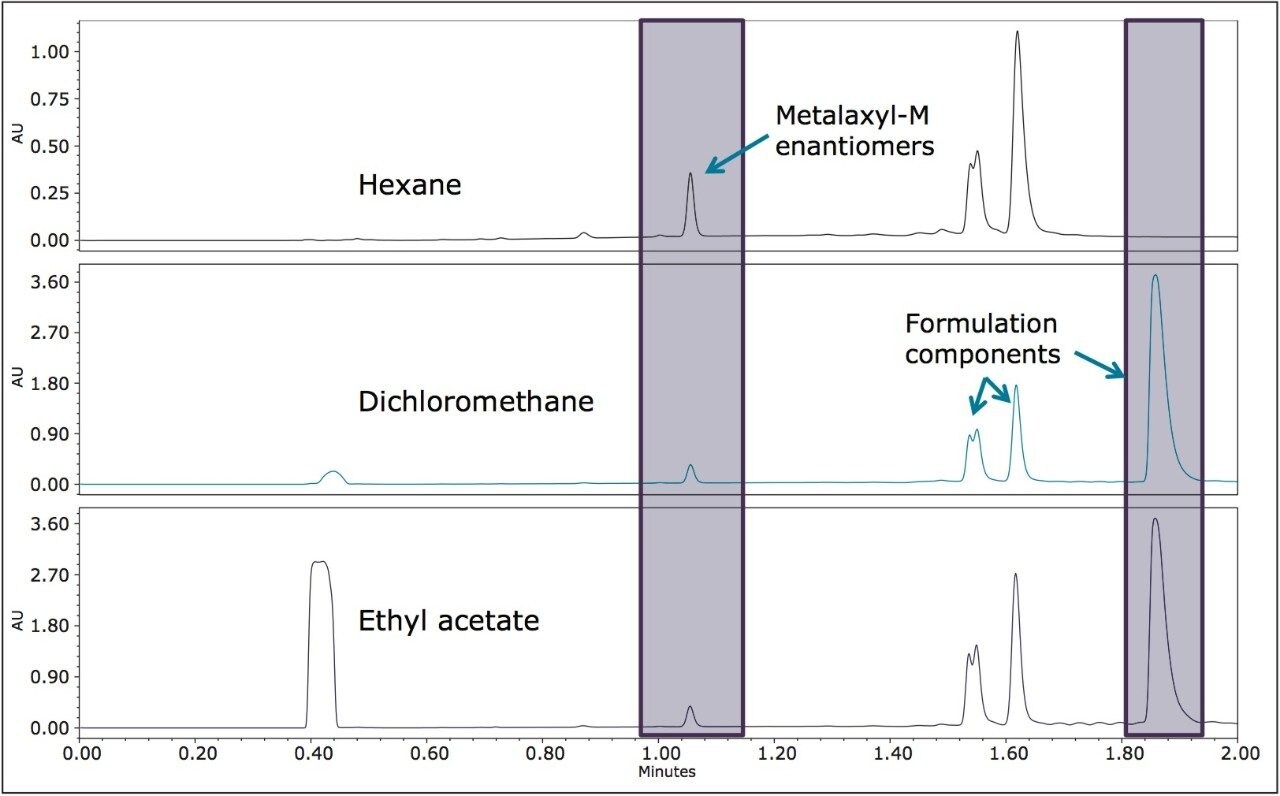
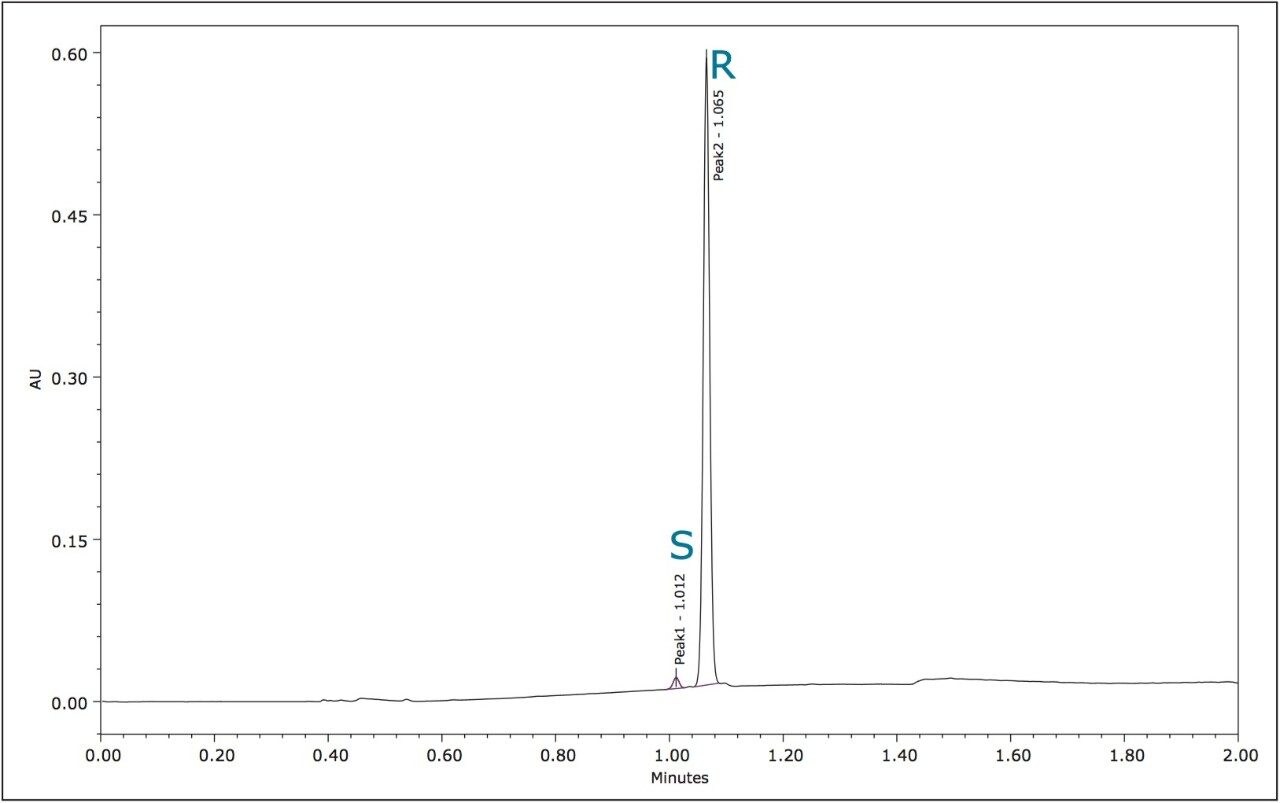
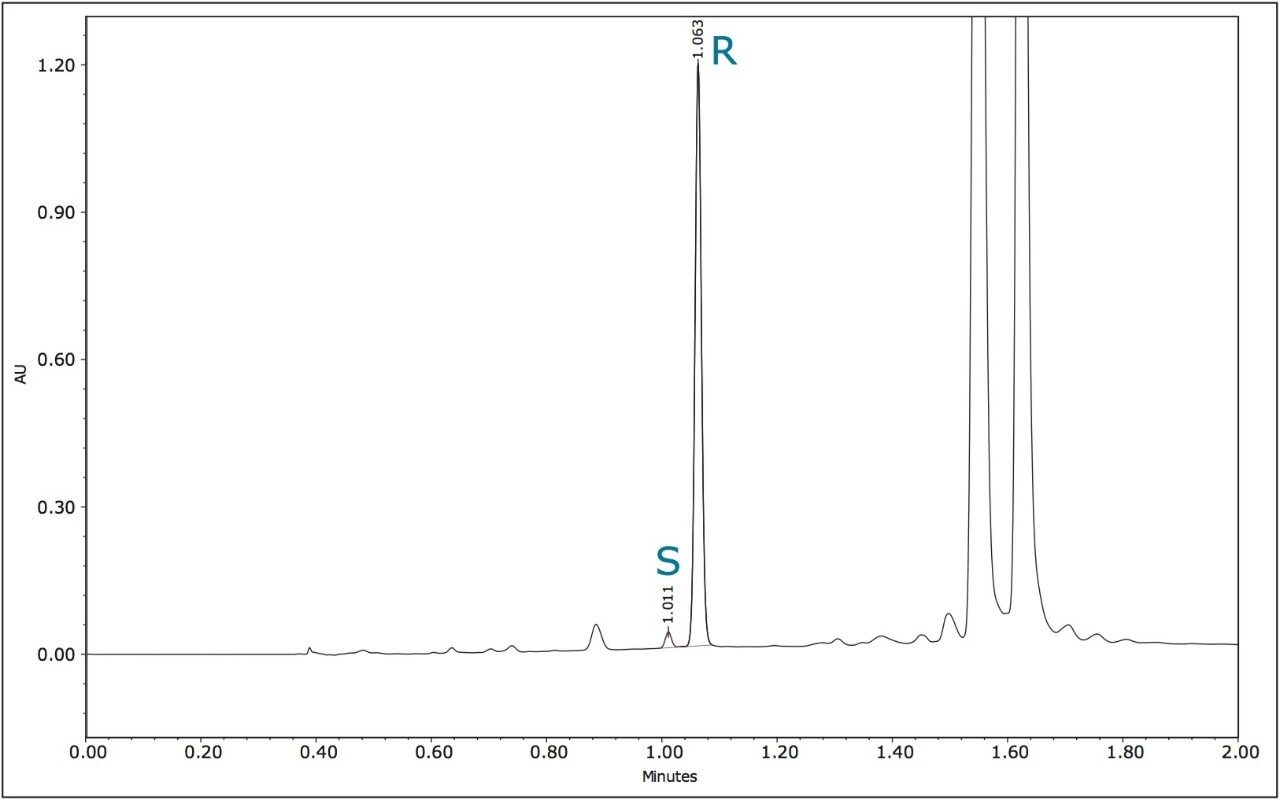
The optimized UPC2 methods allowed increased sample throughput and improved enantiomeric and diasteriomeric resolution. In the case of metalaxyl-M, (Figure 7) both enantiomers eluted in one minute with a resolution (Rs) of 2.47. A comprehensive survey of enantioselective separations of chiral pesticides in the literature, published in 2009, reported a normal phase isocratic method using 60:40 hexane/ethanol for the resolution of metalaxyl enantiomers.6,13 The Rs was reported to be 1.94 with both enantiomers eluting in just under 15 minutes.
The four stereoisomers of metolachlor eluted in 4.5 minutes (Figure 8) with an Rs of 2.31 between peaks 1,2; Rs of 1.85 between peaks 2,3, and Rs of 1.48 between peaks 3,4. The baseline resolution of the stereoisomers of metolachlor standard using a normal phase separation with 91:9 hexane/diethyl ether was also reported in the 2009 article. A figure from this publication showed all four stereoisomers eluting between 20 and 30 minutes.5,6
In both of the UPC2 methods, the stereoisomers of metalaxyl-M and S-metolachlor were sufficiently resolved from the other formulation components so that accurate and reproducible enantiomeric ratios could be obtained. Measurement of the area % for the two metalaxyl-M enantiomers over 100 injections gave %RSDs of 0.02 for R-metalaxyl and 0.85 for the lower level S-metalaxyl (data not shown).
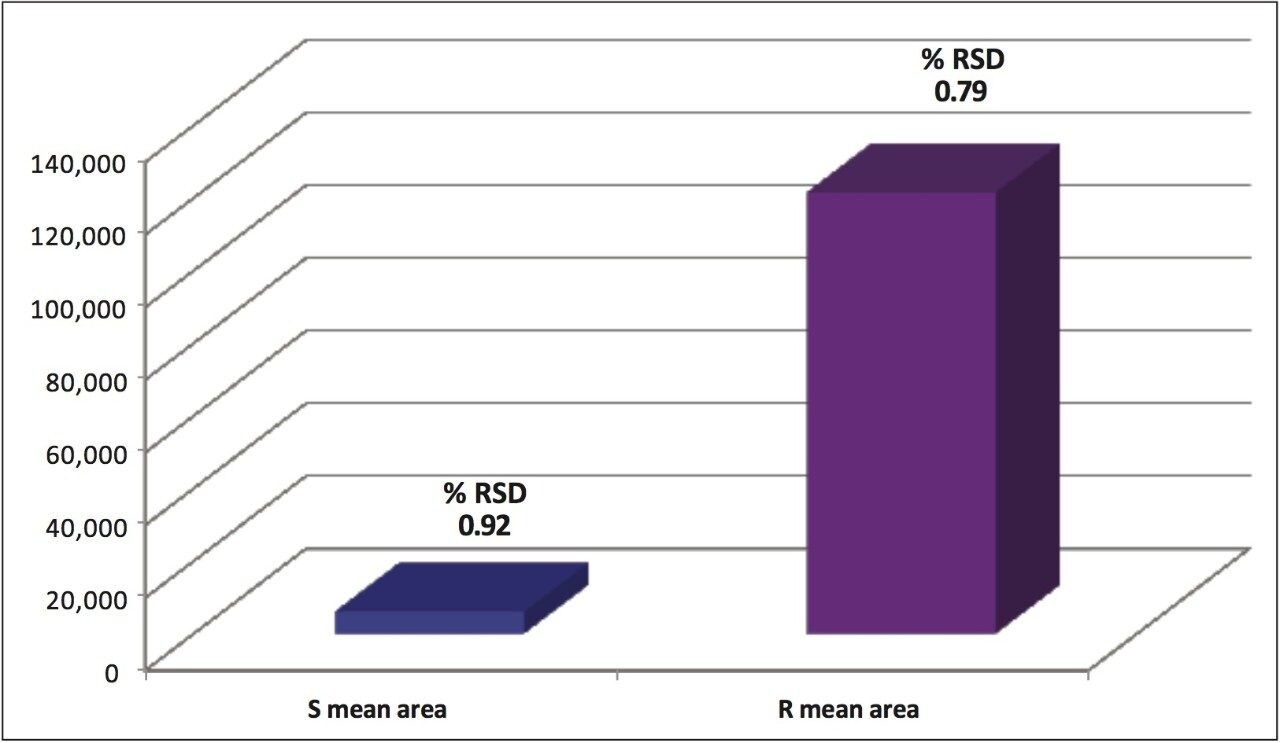
Reproducibility data (n=6) for retention time, area, area %, and height resulted in %RSDs less than or equal to 1.34 for all the stereoisomers of each compound. The bar chart (Figure 9) shows the mean peak areas for the S and R-metalaxyl enantiomers and the %RSDs obtained from six injections. Despite the relative difference between the peak areas for the lower level S-metalaxyl and the active R-metalaxyl, the %RSDs are comparable.
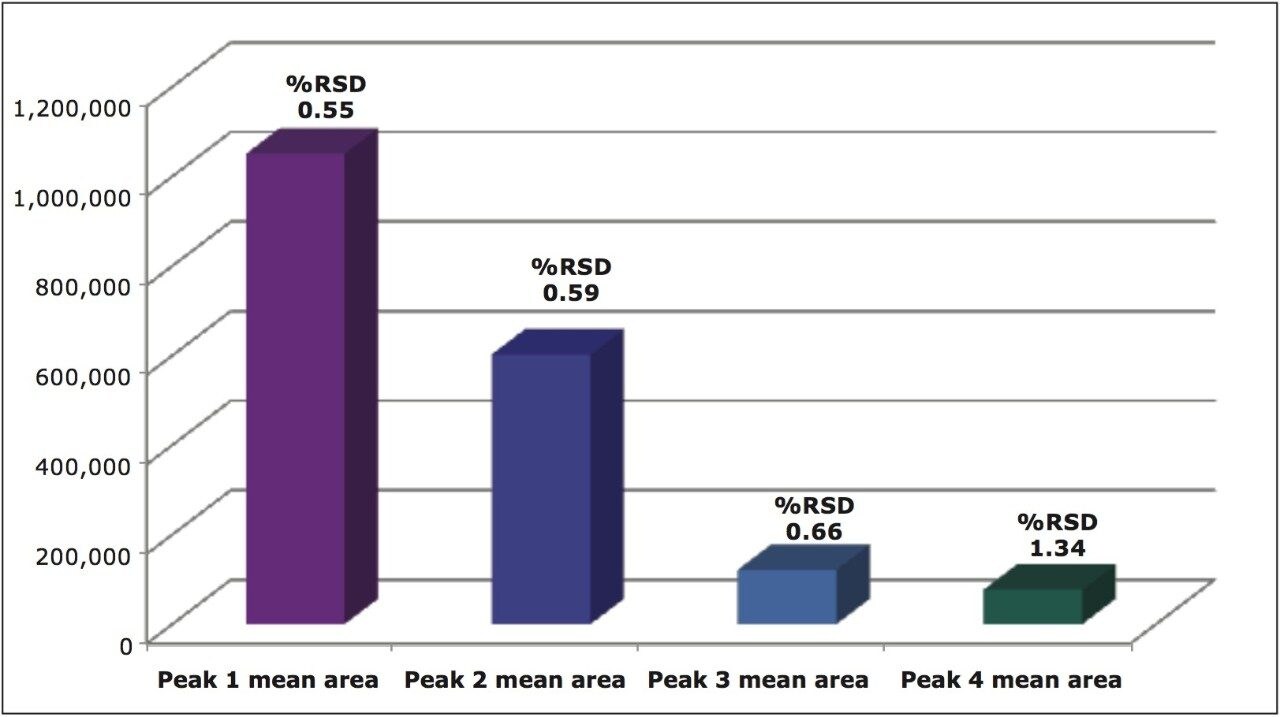
The mean peak areas (n=6) for the four stereoisomers of metolachlor and the %RSDs ranging from 0.55 to 1.34 are shown in Figure 10.
UPC2 allows high efficiency separations that can significantly increase sample throughput. The methods use supercritical CO2 as the primary mobile phase and 2-propanol as the co-solvent.
This application note has demonstrated the chiral analysis of two pesticides in aqueous-based formulations using UPC2. The enantiomer and/or diasteriomer composition in the formulation was measured precisely and accurately using the UPC2 methods that were faster than traditional normal phase methods reported in the literature allowing higher sample throughput.6 The time to develop a chiral separation with acceptable resolution was also decreased as a result of the faster analysis times that are possible using this technique. The %RSDs for peak area, peak height, area %, and Rt were equal to those typically obtained by analyses using UltraPerformance Liquid Chromatography (UPLC)/UV. In addition, the need to use large volumes of potentially hazardous solvents like those used in traditional normal phase chiral separations is reduced as is the cost associated with solvent waste disposal.
The study of chiral pesticides has previously been challenging due to the difficulty in resolving them in short time frames. The ACQUITY UPC2 System overcomes this challenge by providing fast analytical methods to resolve these challenging chiral compound separations. Critical information pertaining to their stereoselective behavior can be obtained more rapidly allowing increased laboratory productivity.
720004744, June 2013