
This is an Application Brief and does not contain a detailed Experimental section.
The Waters ACQUITY UPC2 System utilizes CO2 mobile phases along with organic co-solvent and additives to provide orthogonal selectivity to that of RPLC. For the analysis of vanilla extracts, this separation technique provides greater retention of highly polar, secondary components of vanillin while providing adequate retention and identification of non-polar adulterants. In addition, this chromatographic technique allows for improved efficiency and lower solvent usage than traditional RPLC methods, while providing a high-throughput, sensitive screening method for the analysis of vanilla extracts.
UPC2 Technology provides greater retention of highly polar, secondary components of vanillin while providing adequate retention and identification of potentially harmful, non-polar adulterants.
To reduce the cost of vanilla extract, some manufacturers use synthetic or artificial flavorings in place of more expensive pure vanilla. In many instances, these cheaper alternatives include synthetic components, such as ethyl vanillin. However, some extracts contain potentially harmful adulterants, including coumarin, a fragrance derived from tonka beans. This particular adulterant is a suspected carcinogen, and can interact with blood-thinning medications. While coumarin is banned in the United States for use as a food ingredient, in recent years its prevalence in vanilla extracts has led to consumer warnings from the FDA (2009).1
A number of reversed-phase liquid chromatography (RPLC) methods have been developed for analyses to determine the actual components in vanilla extract.2-4 These methods screen for both synthetic and artificial flavorings as well as secondary vanillin components, the latter of which are indicative of authentic extract from vanilla beans. While these methods can provide high-throughput analyses,4 an orthogonal separation can provide benefits in terms of different selectivity. For example, in reversed-phase separations, some vanillin secondary compounds, such as vanillic acid, are poorly retained, making separation of these polar components challenging.5 In convergence chromatography, the elution of components is reversed, allowing for greater retention and resolution of highly polar compounds.
Method development was performed using a standard containing flavor components from vanilla pods (vanillin, 4-hydroxybenzoic acid, 4 hydroxybenzaldehyde and vanillic acid), a synthetic vanillin (ethyl vanillin), and coumarin, a banned adulterant. The standard was prepared in 2 propanol. A 2.5-minute method was developed using an ACQUITY UPC2 BEH 2- Ethylpyridine 130Å 3.0 x 100 mm, 1.7 μm Column, as shown in Figure 1. The UV method conditions used 20 mM citric acid in methanol as a modifier/additive to improve peak shape for the acidic components.
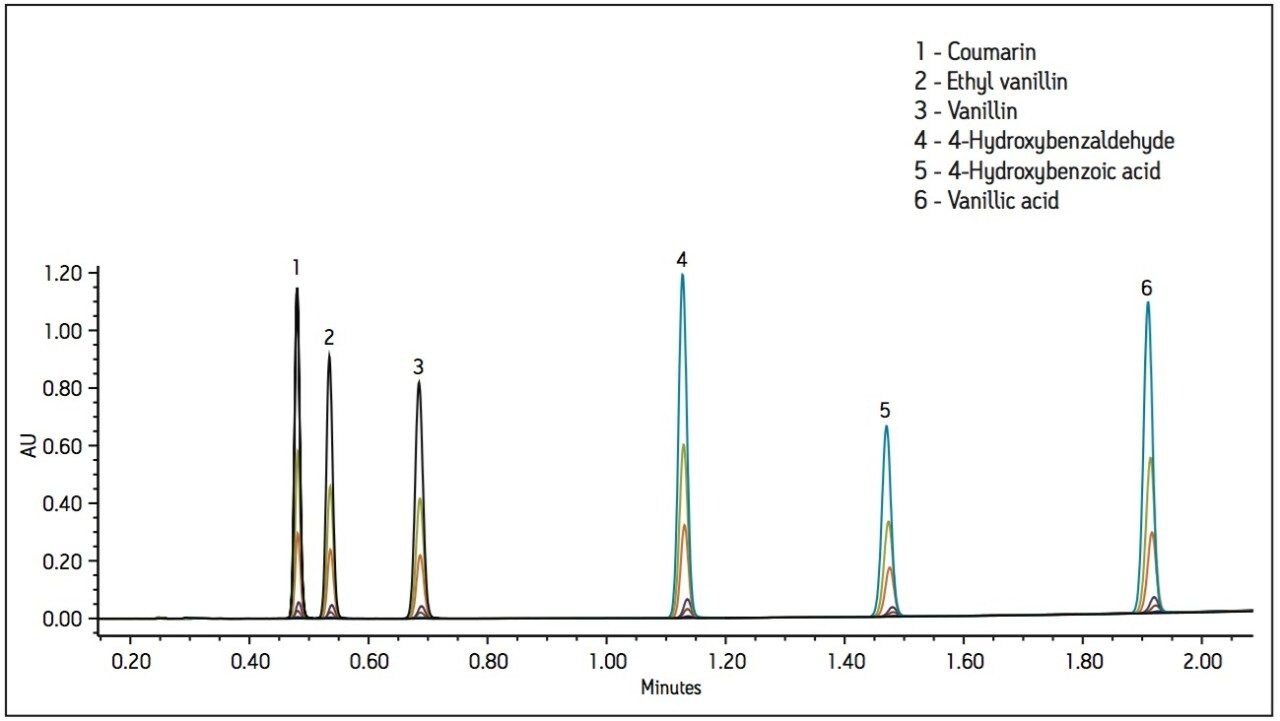
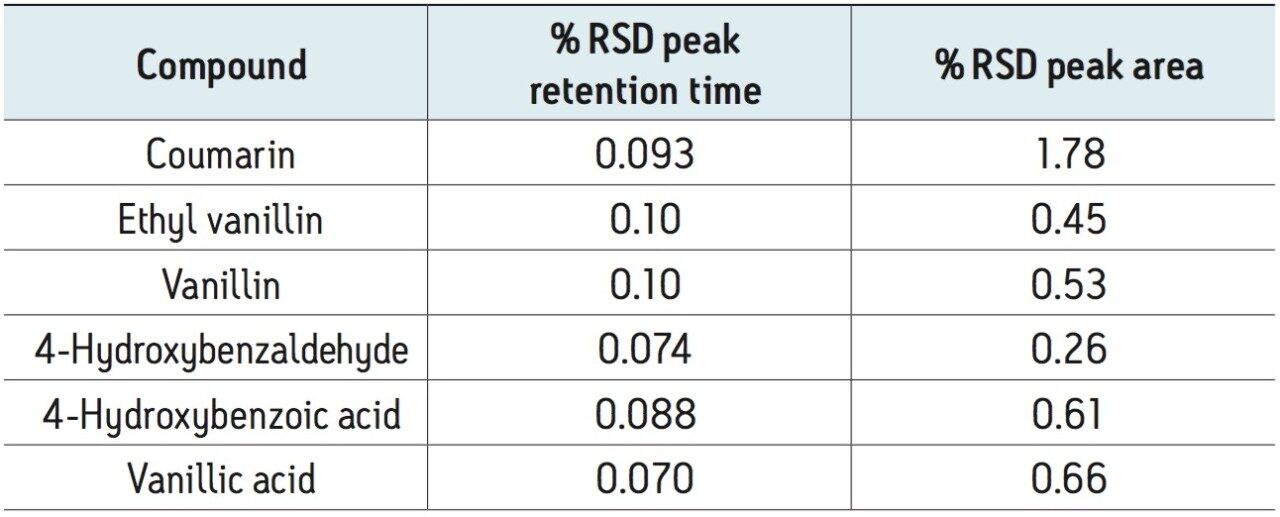
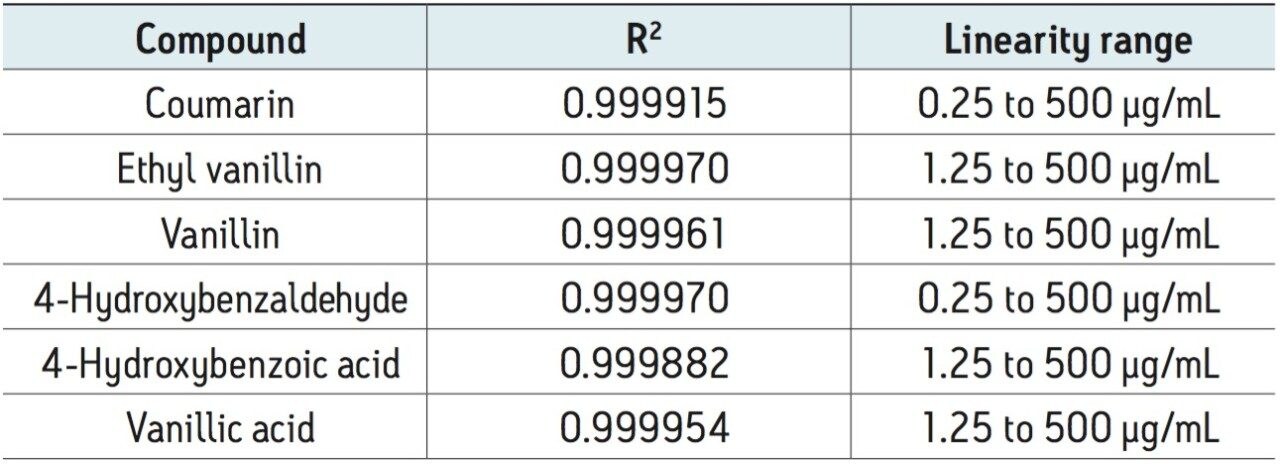
The UV method was evaluated for repeatability (Table 1) and linearity (Table 2). Standards were prepared from 0.250 to 500 μg/mL. Retention time repeatability (n=5) at 12.5 ug/mL was ≤0.10 %RSD and peak area repeatability for the same standard injections was <1.80 %RSD. Linearity was demonstrated between two to three orders of magnitude, analyte-specific, with R2 values >0.999 (Table 1). The limit of quantitation (LOQ) for the tested analytes ranged from 0.250 to 1.25 μg/mL. Given that the analysis of vanilla extracts requires dilution of the sample, the sensitivity requirements for this particular assay were met using the UV method.
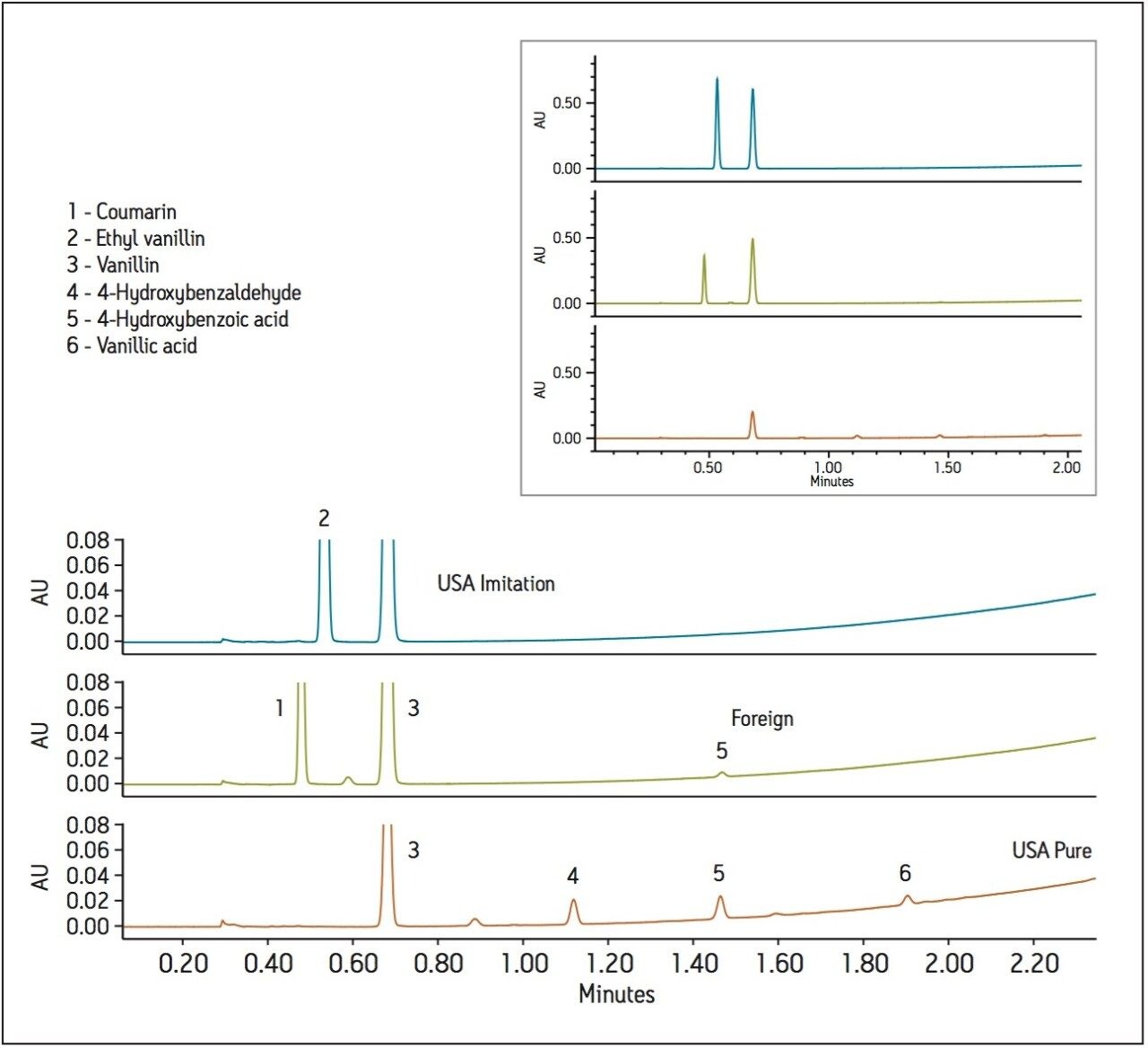
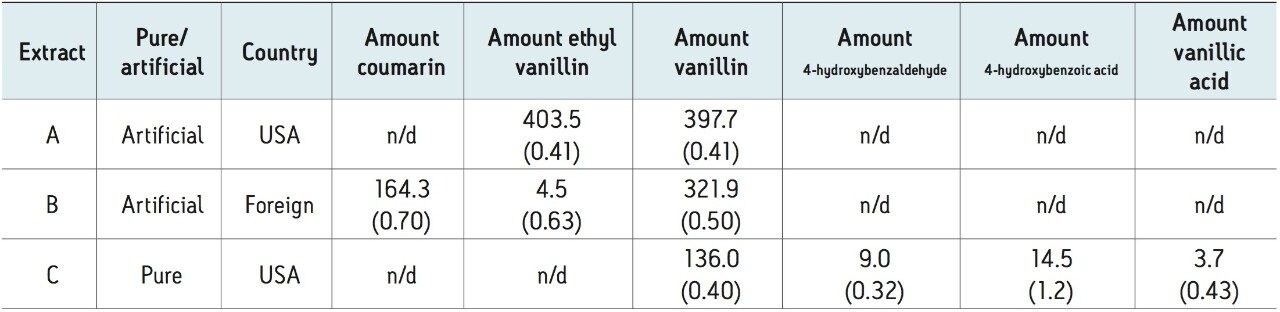
To test for adulteration, the method was used to screen vanilla extracts including those labeled both pure and imitation, from different geographical regions (Figure 2). The vanilla extracts were diluted 10X in ethanol (for sample miscibility), and filtered prior to analysis. Analysis of the imitation vanilla extract from the United States (A) showed the presence of both synthetic vanillin (ethyl vanillin) and vanillin. The absence of other natural flavor components in this sample indicated that the vanillin was likely from a synthetic source. A known imitation vanilla extract purchased outside the United States (B) contained both the adulterant coumarin as well as vanillin, again likely from a synthetic source due to the absence of the secondary vanilla components. Lastly, analysis of a labeled “pure” vanilla extract (C) confirmed itsauthenticity. Vanillin, as well as secondary natural flavor components, were identified and quantified in this sample. In addition, the ratio of vanillin to 4-hydroxybenzaldehye (14.9) was within the previously indicated range for authentic vanilla extracts (Table 3).2
The Waters ACQUITY UPC2 System utilizes CO2 mobile phases along with organic co-solvent and additives to provide orthogonal selectivity to that of RPLC. For the analysis of vanilla extracts, this separation technique provides greater retention of highly polar, secondary components of vanillin while providing adequate retention and identification of non-polar adulterants. In addition, this chromatographic technique allows for improved efficiency and lower solvent usage than traditional RPLC methods, while providing a high-throughput, sensitive screening method for the analysis of vanilla extracts.
720004701, May 2013