
This application note describes a sensitive method for the simultaneous determination of eight endocrine-disrupting compounds in river water samples based on SPE followed by LC-MS/MS.
Emerging evidence from wildlife and laboratory studies indicates that some chemicals may interfere with the endocrine system. Compounds identified as endocrine-disrupting chemicals (EDCs) include pesticides, polychlorinated biphenyls (PCBs), dioxins, furans, alkylphenols, and steroid hormones (natural and synthetic). The steroid hormones are of special concern due to their potency. The natural sex hormone estradiol and its metabolites (estrone and estriol) and the synthetic steroid ethinylestradiol are excreted in the urine of mammals and can be found in surface and ground waters. Other EDCs, such as the alkylphenols–nonylphenol, bisphenol A- and pentachlorophenol are derived from industrial and domestic activities and also occur in environmental waters. Bisphenol A is used in the production of epoxy resins and polycarbonate plastics which are utilized extensively in the manufacture of food and drink packaging materials. Nonylphenol, which is produced as a derivative of non-ionic surfactants, is used extensively as a plasticizer. Pentachlorophenol is still used in some countries as a heavy-duty wood preservative.
The environmental presence of these compounds highlights the need to develop highly sensitive and specific analytical methods. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a technology applicable to a wide range of molecules and matrices, which provides the sensitivity required for trace analysis. Electrospray ionization (ESI) in negative ion (NI) mode is generally the method of choice for determination of estrogens, alkylphenols and chlorophenols1,2. These chemicals have been shown in laboratory and field studies to provoke endocrine disruption at sub-ng/L levels. Thus, analyte enrichment is sometimes necessary in order to achieve the required detection limits. For pre-concentration of EDCs from aqueous samples, solid-phase extraction (SPE) is considered to be the most appropriate technique3. This application note describes a sensitive method for the simultaneous determination of eight endocrine-disrupting compounds in river water samples based on SPE followed by LC-MS/MS.

The following extraction method was used:4
|
Cartridge: |
Waters Oasis HLB (60 mg) |
|
Conditioning: |
3 mL Methyl t-butyl ether (MTBE) 3 mL Methanol (MeOH) 3 mL ultra-high quality water |
|
Load: |
500 mL acidified river water |
|
Sample: |
(10 mM formate buffer pH=3.0) |
|
Wash: |
3 mL 40% MeOH in UHQ water 3 mL UHQ water 3 mL 10% MeOH/2% NH4OH in water |
|
Elute: |
6 mL 10% MeOH/MTBE |
|
Evaporate: |
to dryness by gentle stream of nitrogen at 50 °C |
|
Re-dissolution: |
500 μL (50:50, v/v, acetonitrile/ ammonium formate buffer pH 3.0) |
|
Waters Alliance 2690 HPLC System |
|
|---|---|
|
Mobile phase A: |
Methanol |
|
Mobile phase B: |
Water |
|
Column: |
SunFire C18, 2.1 x 50 mm with 3.5 μm particle size |
|
Flow rate: |
0.2 mL/min |
|
Injection volume: |
20 μL |
|
Time (min) |
%A |
%B |
|---|---|---|
|
0 |
60 |
40 |
|
10 |
100 |
- |
|
18 |
100 |
- |
|
20 |
60 |
40 |
|
23 |
60 |
40 |
A Micromass Quattro micro Triple Quadrupole Mass Spectrometer was operated in the negative ion electrospray mode. Nitrogen gas, at a flow rate of 450 L/hr and a temperature of 250 °C, was used for spray desolvation. The source temperature was maintained at 120 °C and the capillary voltage was 3.2 kV.
The MRM transitions, along with the optimum cone voltage and collision energy for the individual compound, are listed in Table 1. The ion used for quantitative determinations has been highlighted in bold font. In the case of pentachlorophenol, no significant fragmentation could be obtained even at high collision energy, but the full scan acquisition mode revealed that enough structural information is obtained due to the characteristic isotope ratio signals of the halide. Thus, for this compound, a SIR of four of its characteristic ions have been monitored.
Data was acquired using Waters MassLynx Software and processed using the TargetLynx Application Manager.
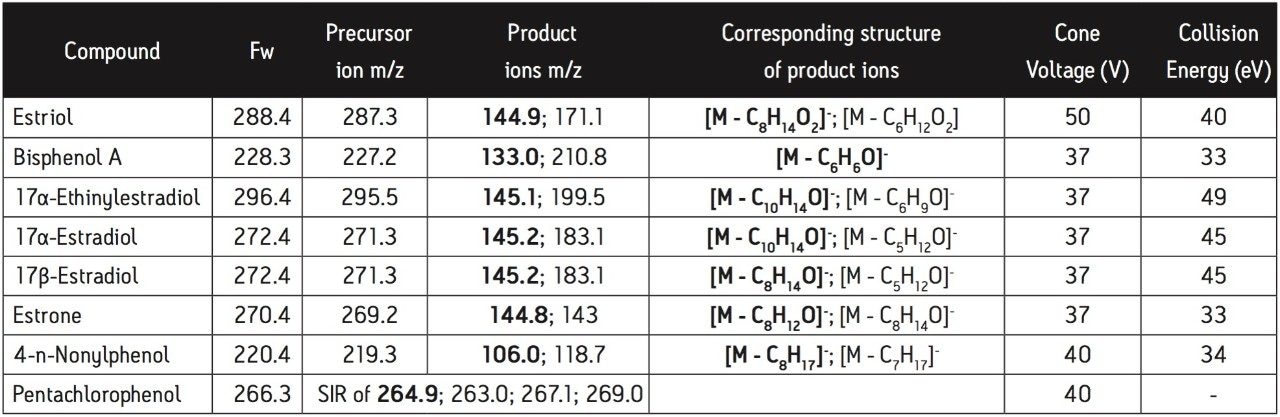
The nature of all eight compounds studied makes them amenable to analysis by electrospray injection (ESI-) MS/MS. Methanol and acetonitrile, two organic solvents commonly used in reversed-phase LC, were evaluated. The MS signals for all the compounds assayed were higher for methanol than for acetonitrile, probably due to the more favorable ionization properties of the former solvent. Calibration models for the LC/(ESI-) MS/MS method were constructed by injecting standard solutions and obtaining a good linear relationship between the analytical signal and analyte concentration for all compounds. Figure 1 shows a representative calibration curve for estrone generated in the concentration range of 0.5–100 ng/mL. This calibration curve was generated using solvent standards and the concentrations stated refer to the analyte concentration in the sample vial.
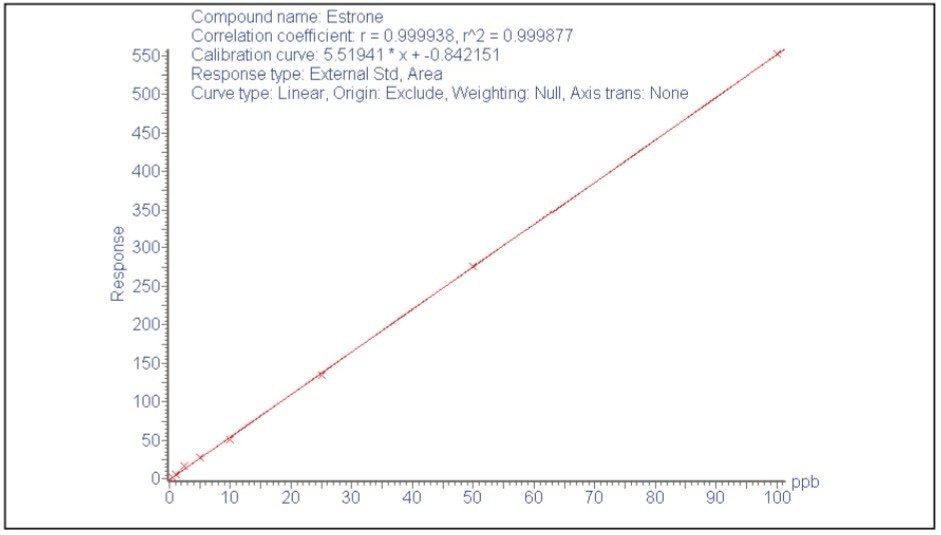
Figure 2 shows the chromatograms for the compounds at the lowest calibrated level. The instrument LoDs in solvent standard (S/N ratio = 3 to 1) are estimated to be 0.6 ng/mL for estrone, 0.5 ng/mL for pentachlorophenol, 3 ng/mL for 17α-estradiol, 5 ng/mL for |17β-estradiol, 10 ng/mL for bisphenolA, 7 ng/mL for 4-n-nonylphenol, 5 ng/mL for estriol, and 30 ng/mL for 17α-ethinylestradiol.
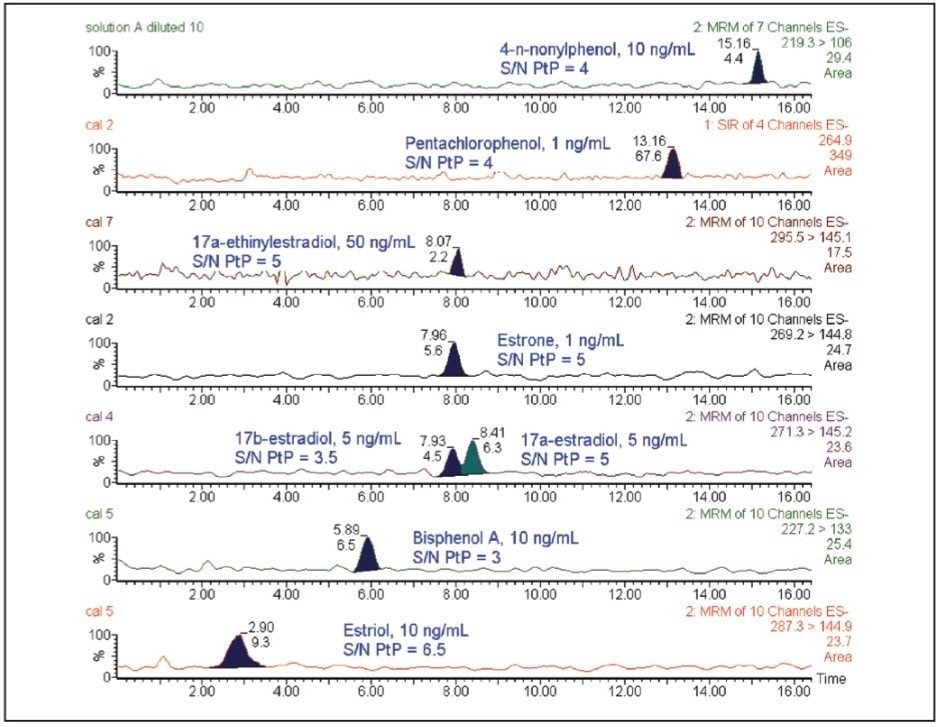
In order to obtain a more sensitive method for the quantification of these compounds in river water, a solid-phase extraction step with Oasis HLB cartridges was performed prior to chromatographic determination. A study with different volumes of river water samples was carried out first to evaluate if effects of matrix suppression or breakthrough appear when the volume of water to be pre-concentrated was increased. The results show that the signals were practically independent of the pre-concentrated sample volume. Accordingly, a 500 mL river water sample volume was selected. With the proposed SPE procedure, satisfactory percentage recoveries in river water were obtained ranging from 74% for 4-n-nonylphenol to 105% for 17α-ethinylestradiol.
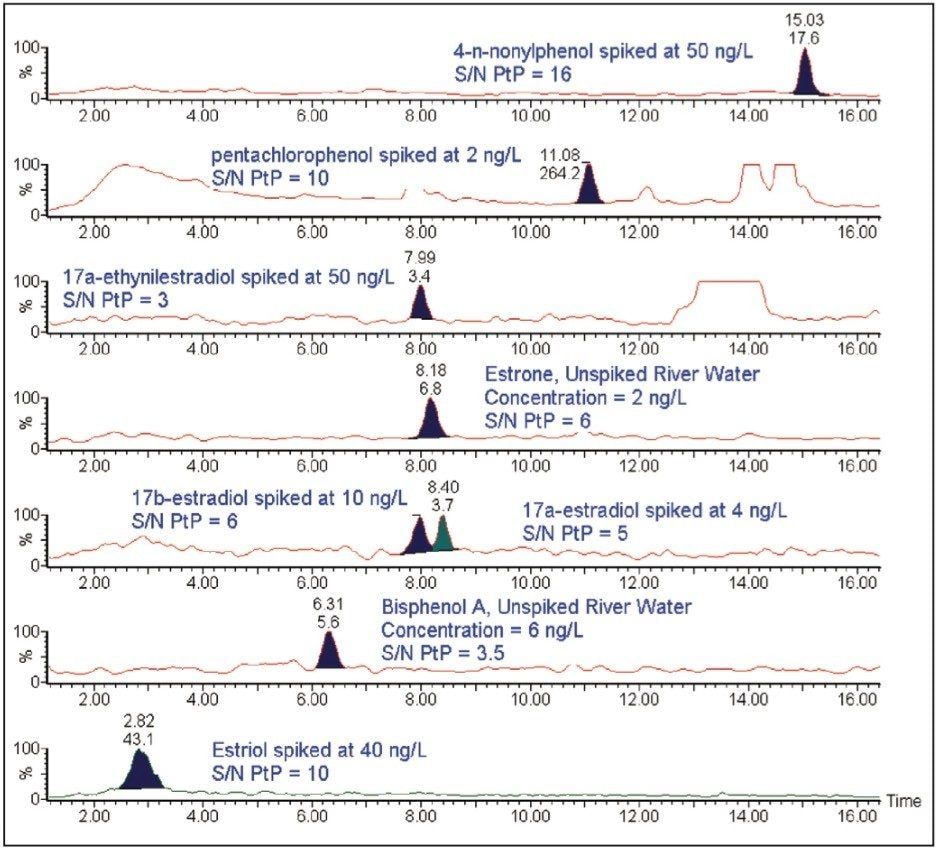
The whole procedure was applied to river water samples. The analysis of unspiked river water samples revealed that two of the compounds studied, bisphenol A and estrone were present, as can be seen in Figure 3.
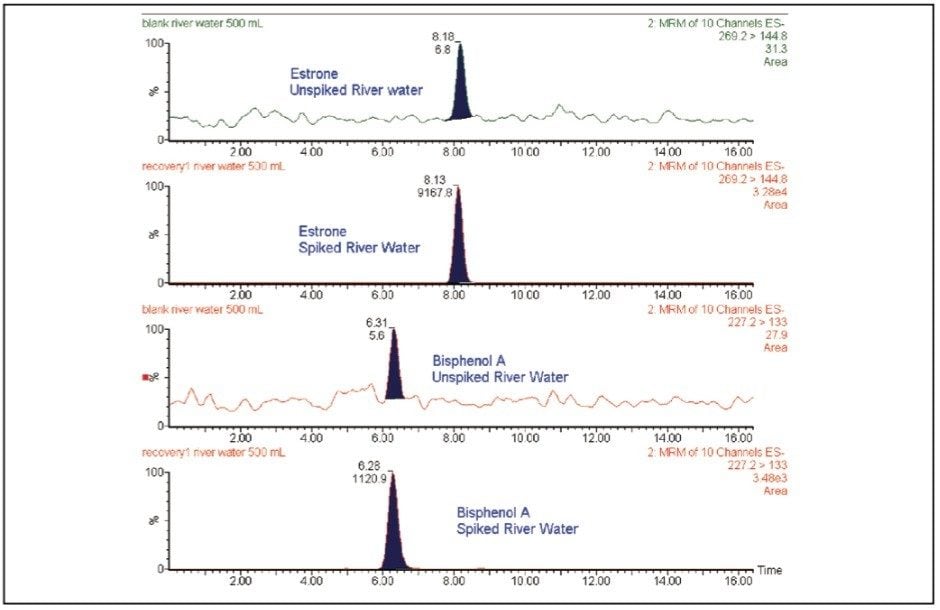
To estimate the concentration of these two compounds in the river water sample, a standard addition method was performed. For the standard addition experiments, addition of known amounts of target analytes into the sample at four concentrations levels (1, 2, 4, and 8 ng/L for estrone and 0.25, 5, 10, and 20 ng/L for bisphenol A) was carried out. From the calibration curves obtained, the concentration found in river water is estimated to be 2 ±1 ng/L for estrone and 6 ±1 ng/L for bisphenol A.
Figure 4 shows the chromatograms obtained after the SPE extraction, for river water samples spiked with the compounds at the lowest concentration level tested (except for bisphenol A and estrone for which chromatograms in unspiked river water are presented). From Figure 4, the method LoDs in river water (S/N ratio = 3 to 1) are estimated to be 1 ng/L for estrone, 0.6 ng/L for pentachlorophenol, 2.5 ng/L for 17α-estradiol, 5 ng/L for 17β-estradiol, 6 ng/L for bisphenol A, 9 ng/L for 4-n-nonylphenol, 12 ng/L for estriol, and 50 ng/L for 17α-ethinylestradiol. These estimates refer to the concentration of analyte spiked into the river water prior to extraction.
A sensitive and selective LC-MS/MS method has been developed for the determination of eight endocrine-disrupting compounds in river water samples at low-nanogram per liter level. The 1000-fold concentration step in the sample preparation method improves the sensitivity of the analysis by 500 to 1000 fold, depending on analyte. This indicates that the SPE method used gives excellent analyte enrichment and cleanup, with minimal matrix effects.
720001296, June 2007