Automated Sample Preparation Using Andrew+™ Pipetting Robot Configured with Extraction+ for Solid Phase Extraction (SPE) of Per- and Polyfluoroalkyl Substances (PFAS) in Food Packaging Material Analysis
Abstract
Per-and polyfluoroalkyl substances (PFAS) are used in many applications, and one of them is as grease-proofing agents in food packaging materials. PFAS can migrate into food from food packaging materials, posing risk to our health via diet exposure. This has raised public concern and regulatory actions are starting to be implemented. In this work, we have established a robust LC-MS/MS method for quantitative analysis of PFAS compounds in food packaging materials using the Xevo™ TQ-Absolute Mass Spectrometer, with good linearity for the 27 PFAS analytes, with r2 values all >0.995. Sample preparation involved solid phase extraction (SPE) to clean the sample matrices using Oasis™ WAX for PFAS cartridges and matrix effects were minimal after employing the SPE workflow. The SPE workflow was fully automated by the Andrew+ Pipetting Robot configured with the Extraction+ connected device to increase the efficiency of the sample preparation process. The SPE workflow was tested by spiking in 14 labelled PFAS analytes to a food packing material and recoveries ranged from 93.6 to 126.6%. The overall workflow was tested on five food packaging materials (1 packaging paper, 3 paper box and 1 paper bag) with a range of PFAS detected in all materials, the highest containing 12 native PFAS.
Benefits
- Maximize your laboratory productivity and efficiency with an automated pipetting and SPE protocol using the Andrew+ pipetting robot and Extraction+ connected device
- Mitigate error in standards and SPE sample preparation with an automated protocol that requires less human intervention
- Achieve low levels of detection as low as 1 pg/dm2 using an ACQUITY™ UPLC™ I-Class Plus coupled to a Xevo TQ-Absolute Mass Spectrometer
Introduction
Per-and polyfluoroalkyl substances (PFAS) are used widely in many commercial applications due to their properties and chemical stability. Due to their widespread use and persistence, these toxic chemicals accumulate in the environment, humans, and animals. One of the major sources of PFAS exposure is through diet, and PFAS which are used in food packaging materials as grease-proofing agents is a potential source for dietary exposure.1 With their unique amphiphilic property, PFAS can easily migrate from food packaging into food depending on the material type, temperature and carbon chain length.2 This has led to public concern and regulatory bodies have started to take action. In 2020, Denmark started to prohibit selling any food contact paper and board materials containing PFAS. With the increasing concern on PFAS, there is more focus on potential contamination sources. Food packaging can come in a wide variety and range which will be challenging especially with the different kind of interferences that might be present.
The preparation of samples including solid phase extraction (SPE) can be time-consuming that can dominate an analyst’s time in the laboratory. With the addition of Andrew+ pipetting robot configured with the Extraction+ connected device, it allows an automated laboratory preparation system to provide the flexibility of freeing analysts’ time for other important tasks, resulting in a more efficient way of time management.
In this application note, an entire workflow is presented for PFAS analysis in food packaging samples from automated sample preparation using Andrew+ Pipetting Robot configured with Extraction+ connected device to LC-MS/MS quantitative analysis with Xevo TQ-Absolute Mass Spectrometer.
Experimental
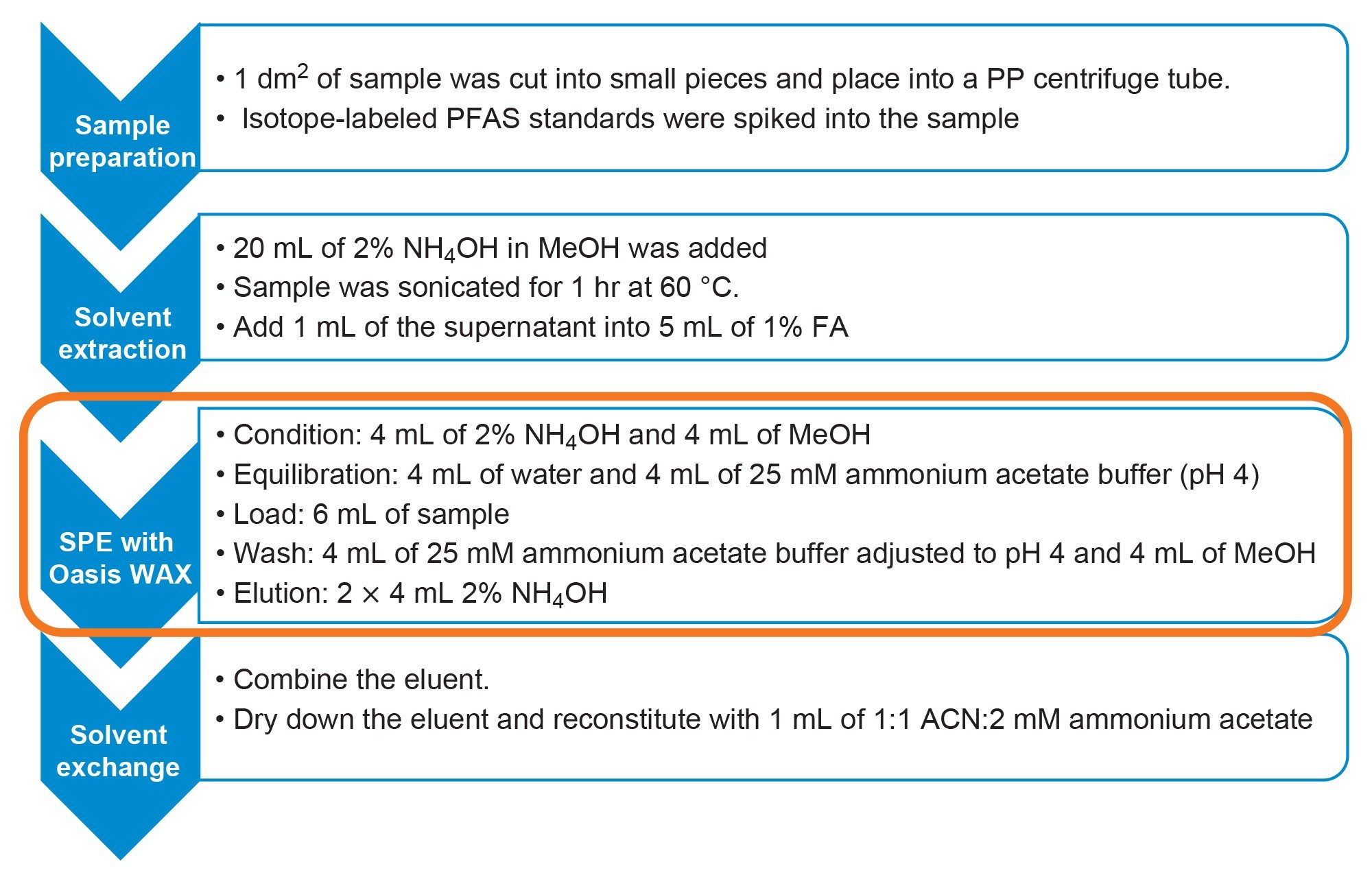
One of the main factors that may affect the analysis is matrix effect due to presence of different interferences in a variety of food packaging materials. To ensure matrix effect is minimized, proper sample clean-up was done using Oasis WAX for PFAS SPE cartridges. The SPE workflow was fully automated using the Andrew+ pipetting robot with Extraction+. The design and execution of protocol was done through OneLab™ Software. It is an intuitive software which allows user the full control of vacuum pressure setting, thus eliminating the need for user intervention in the procedure.
PFAS native standards and isotope-labeled standards were obtained from Wellington Laboratories. An internal standard calibration series in 1:1 ACN:2mM ammonium acetate with concentration ranging from 0.001–1 ng/mL was prepared using the Andrew+ Pipetting Robot.
An area of 1 dm2 from a food packaging sample was cut into small pieces (ca. 1 cm2) and placed in a 50 mL polypropylene centrifuge tube. 50 µL of 10 ng/mL of internal standards were spiked into the sample and left to stand for an hour. Additionally, 20 mL of 2% ammonia hydroxide in methanol was added into the sample and sonicated at 60 oC for 60 minutes. Finally, 1 mL of the sample was added into 5 mL of 1% formic acid solution (aq).
The sample was then subjected to solid phase extraction using the Oasis WAX for PFAS SPE Cartridge (p/n: 186009345). The Andrew+ Pipetting Robot configured with the Extraction+ connected device was used to automate the SPE protocol as shown in Figure 1. The protocol was designed and executed using the cloud-native OneLab Software.
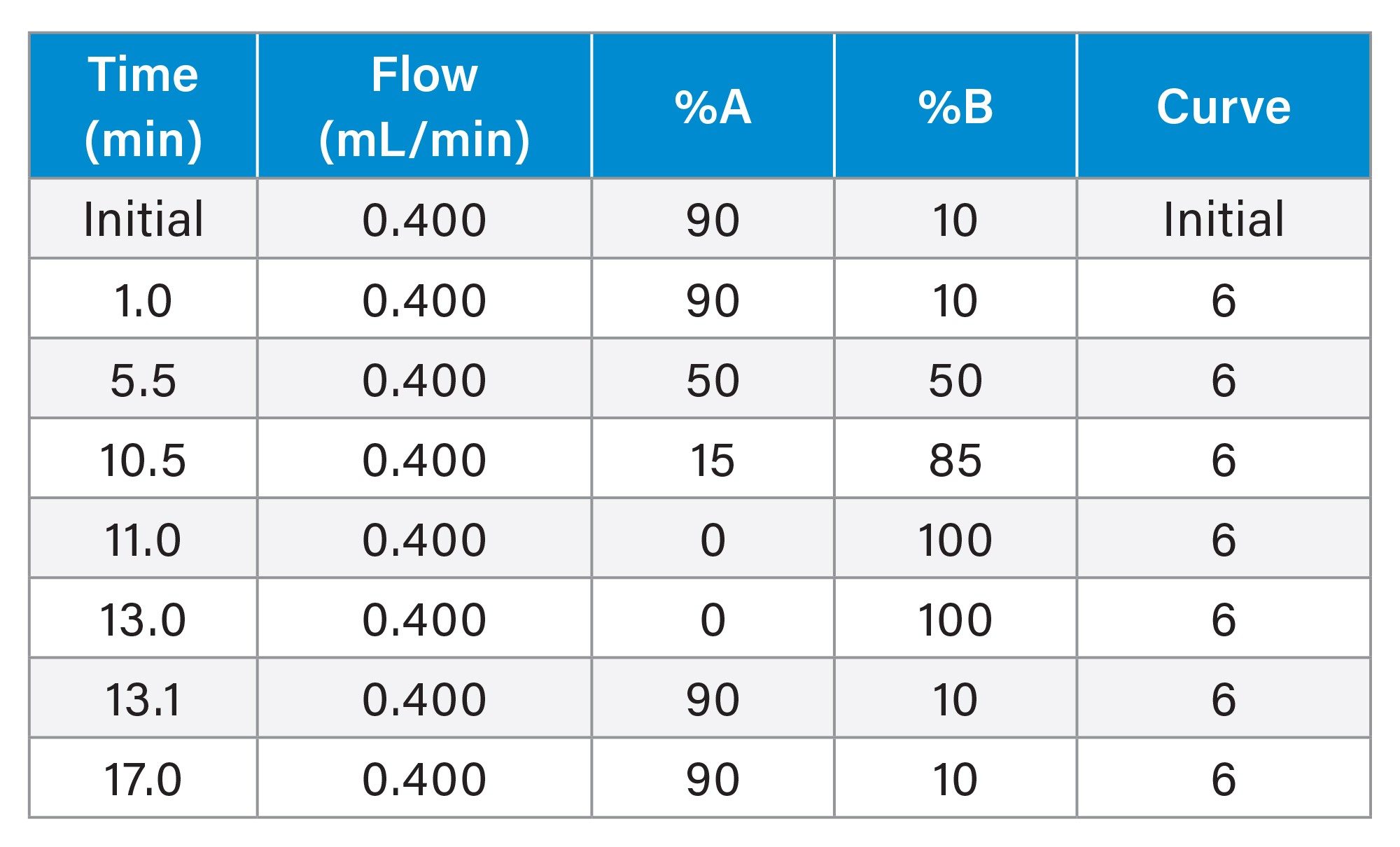
LC Conditions
|
LC system: |
ACQUITY I-Class with FTN modified with PFAS kit |
|
Column(s): |
ACQUITY Premier BEH™ C18 Column, 2.1 x 100 mm, 1.7 µm (p/n: 186009453) |
|
Isolator column: |
Atlantis™ Premier BEH C18 AX, 2.1 x 50 mm, 5 µm (p/n: 186009407) |
|
Column temperature: |
40 °C |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
2 mM Ammonium acetate in water |
|
Mobile phase B: |
2 mM Ammonium acetate in ACN:water (98:2) |
|
Injection volume: |
10 µL |
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ-Absolute Mass Spectrometer |
|
Ionization mode: |
ESI (-) |
|
Capillary voltage: |
1 kV |
|
Source temperature: |
130 °C |
|
Desolvation temperature: |
550 °C |
|
Cone gas flow: |
150 L/Hr |
|
Desolvation gas flow: |
1000 L/Hr |
Data Management
|
Chromatography software: |
MassLynx™ v4.2 |
|
MS software: |
MassLynx v4.2 |
|
Informatics: |
TargetLynx™ v4.2 |
Results and Discussion
PFAS internal standard calibration series
The preparation of internal standard calibrants is time consuming and involves a lot of repetitive pipetting steps. The use of Andrew+ Pipetting Robot to automate the protocol helps reduce potential human error and allows analysts to attend to more important tasks.

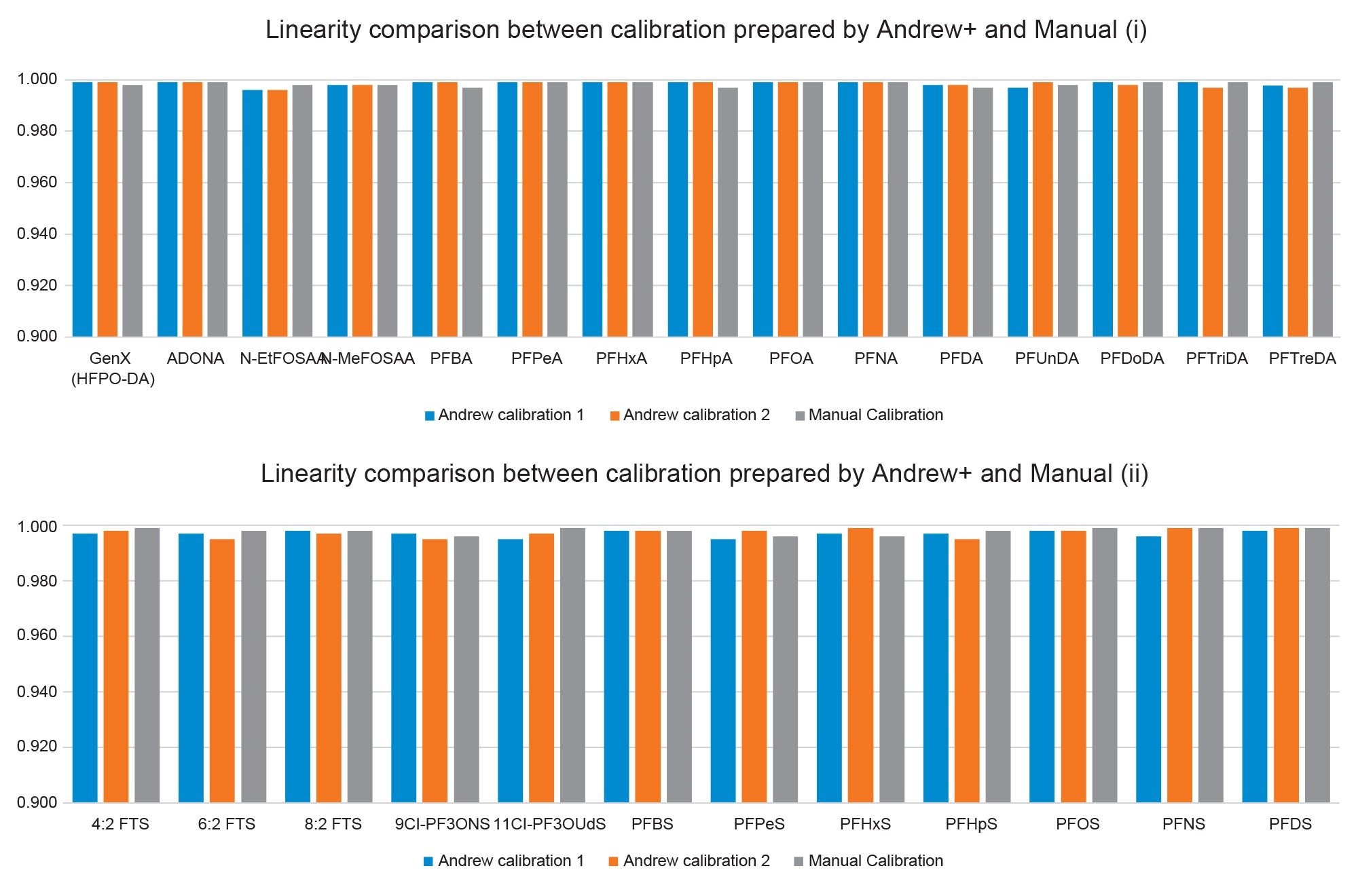
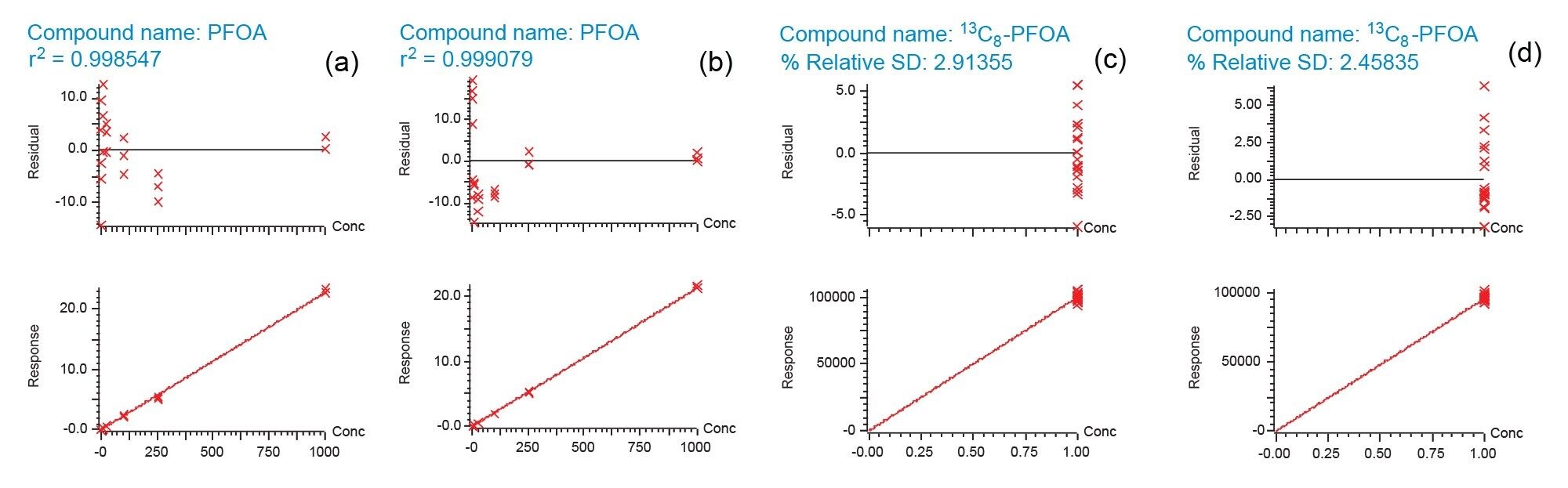
The calibration series for 27 PFAS compounds with concentration range of 1–1000 pg/mL (1–1000 pg/dm2) was prepared on two different batches. The results in Figure 3 show that the linearity of all the PFAS compounds for the two automated batches are reproducible with r2>0.995. A set of calibration series was also prepared by a skilled lab user as a comparison. From Figure 3, the results generated by Andrew+ automated protocol and manual preparation are comparable. Using PFOA as an example, the calibration curve and residual plot as shown in Figure 4 further suggest that similar results can be obtained from both ways of preparation.
Recovery and matrix effect studies
As it is difficult to find food packaging that is PFAS-free, the recovery and matrix effect studies were evaluated using 14 isotope-labeled PFAS standards spiked in food packaging matrix. The SPE protocol with Oasis WAX for PFAS was fully automated by the Andrew+ Pipetting Robot configured with Extraction+, controlled by Onelab, a cloud-native software.
OneLab Software is an easy-to-use software with an intuitive interface which allows the user to get started fast in designing and executing protocols without programming knowledge. It enables the full control of the vacuum pressure, allowing the user to set different pressure profiles in protocols according to the solvents used during the SPE steps. This eliminates the need for the user to control the solvent flow manually, which is critical in achieving good recovery.

Brown food wrapping paper was used for evaluation of recovery and matrix effects. Three batches of sample were evaluated with one batch extracted with manual SPE protocol while two other batches were extracted with automated SPE protocol. Good recoveries and matrix effects for all the compounds were obtained ranging from 85.2%~121.8% and 78.0%~127.3% respectively.
Table 1 shows that the results are reproducible from the same sample type prepared in two separate batches. Also, manual SPE protocol and automated SPE protocol yield almost similar result.
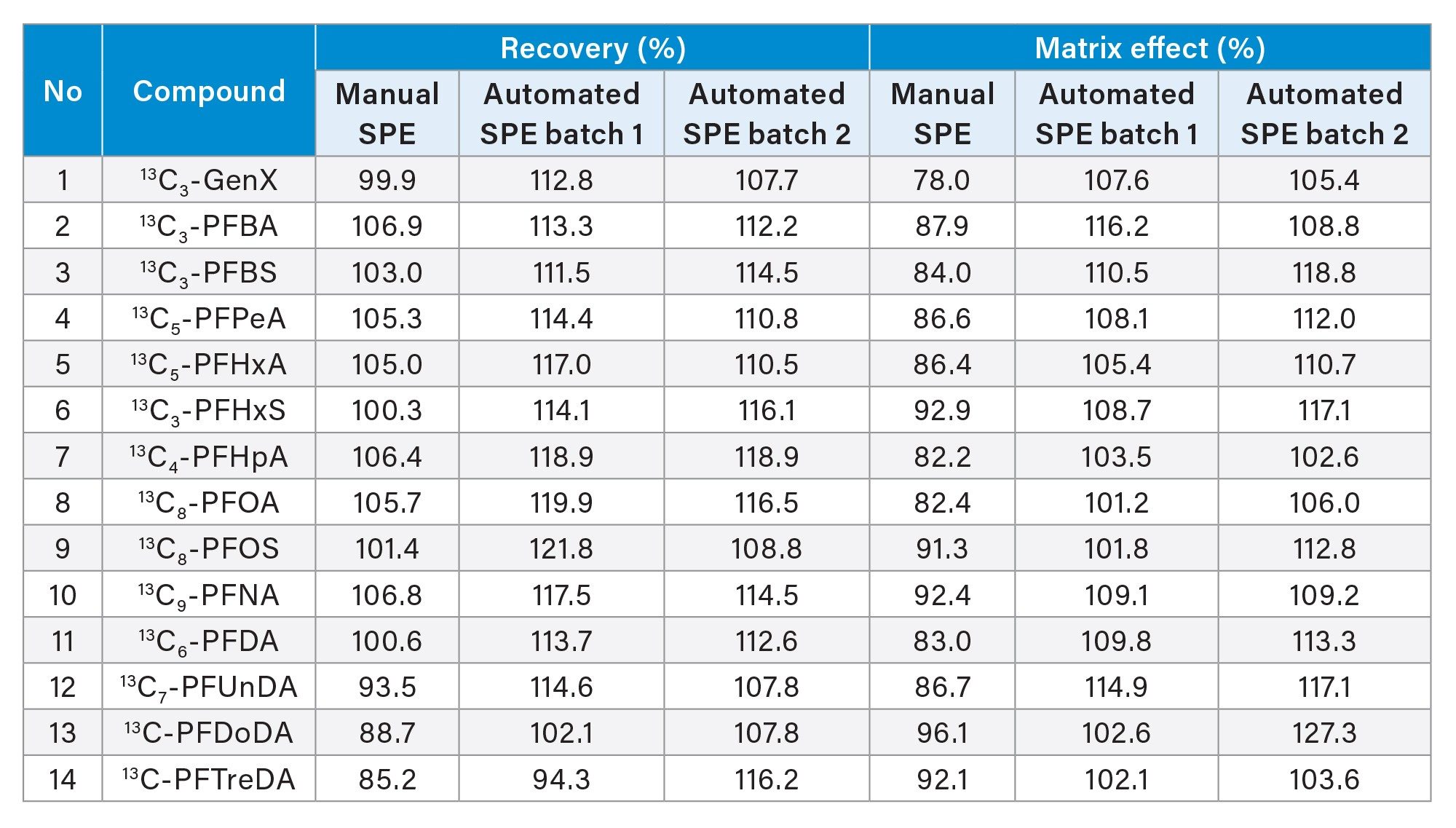
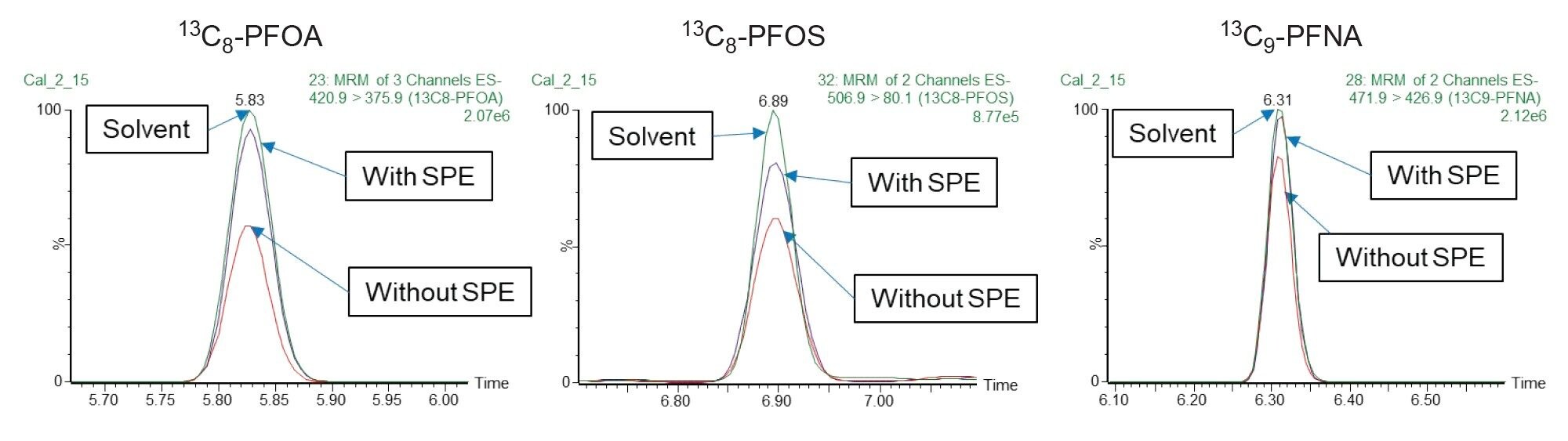
Food packaging matrices are complicated, and matrix effects will vary with each sample. Matrix effect is determined by peak area of PFAS prepared in solvent divided by peak area of PFAS prepared in blank sample extracts. Some matrices may cause ion suppression (Matrix effect <80%) which will reduce the sensitivity of detection of native PFAS in samples. This will lead to inaccuracy in determining the right amount of PFAS present in the sample. SPE clean-up helps remove unwanted matrix, giving a cleaner sample and better sensitivity.
Figure 6 shows the comparison of MRM chromatograms of a sample with and without the SPE clean-up procedure. With SPE clean-up, the sensitivity of 13C8-PFOS, 13C8-PFOA and 13C9-PFNA improves significantly.
Detection of Native PFAS in Samples
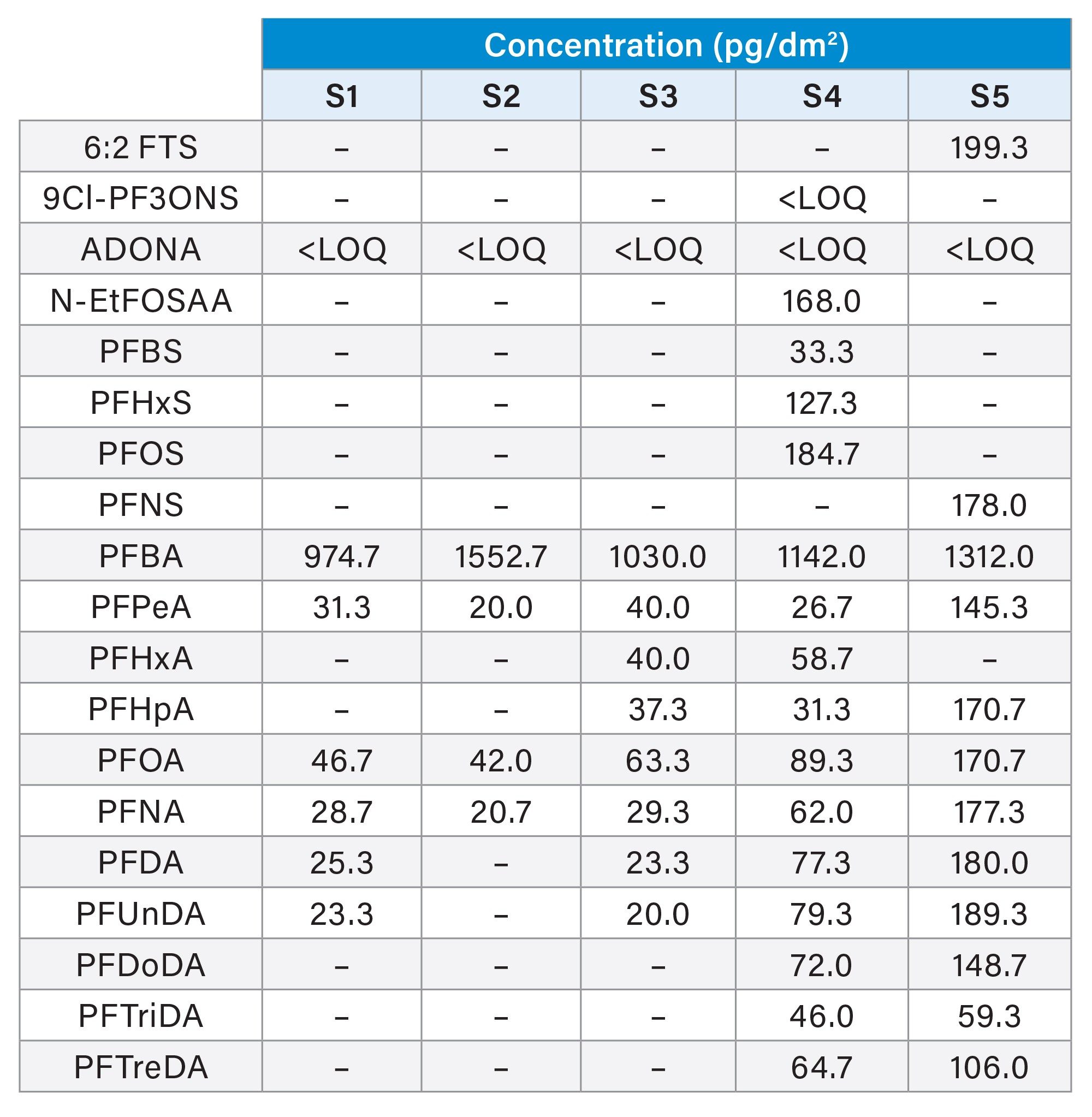
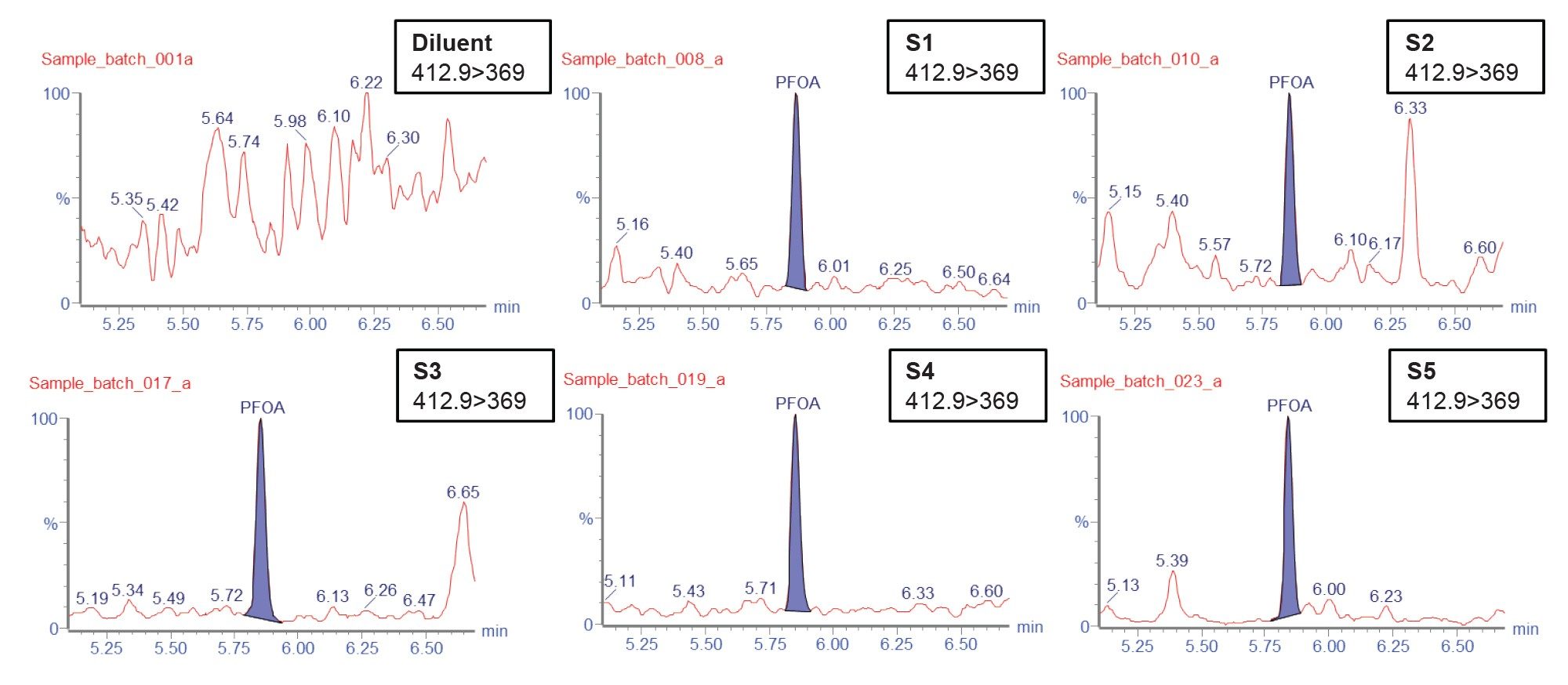
Five food packaging samples (1 packaging paper, 3 paper box, and 1 paper bag) obtained from the market were analyzed using the established LC-MS/MS method. These packaging materials are usually used for oily or greasy food. PFAS compounds were found in all five of the samples. The number of PFAS compounds found in each of the sample along with the detected concentration is illustrated in Table 2. There are more than 12 native PFAS compounds detected in samples S4 and S5. PFOA is detected in all samples, which is illustrated in Figure 8.
Conclusion
This work demonstrates the entire workflow of PFAS analysis in food packaging samples from extraction, automated SPE clean-up using Andrew+ pipetting robot configured with Extraction+ connected device, to LCMS quantitative analysis with the Xevo TQ-Absolute Mass Spectrometer. The results suggest the possibility of incorporating automation into the workflow for calibration curve preparation and fully automated SPE protocol instead of repetitive manual tasks. This can help to maximize efficiency by freeing up the time needed and eliminates the need for human intervention in the SPE procedure. The methodology also allows for an easy and highly sensitive analysis of PFAS in food packaging material to better monitor sources that could potentially bring PFAS to our food.
References
- Sapozhnikova, Y.; Taylor, R. B.; Bedi, M.; Ng, C. Assessing Per- and Polyfluoroalkyl Substances in Globally Sourced Food Packaging. Chemosphere 2023, 337, 139381.
- Schwartz-Narbonne, H.; Xia, C.; Shalin, A.; Whitehead, H. D.; Yang, D.; Peaslee, G. F.; Wang, Z.; Wu, Y.; Peng, H.; Blum, A.; Venier, M.; Diamond, M. Per- and Polyfluoroalkyl Substances in Canadian Fast Food Packaging. Environmental Science and Technology Letters 2023.
- LBK nr 1033 af 05/07/2023, Ministeriet for Fødevarer, Landbrug og Fiskeri. Retsinformation. https://www.retsinformation.dk/eli/lta/2023/1033 (accessed 2024-01-31).
720008659, March 2025