Bioanalysis of Sitagliptin and Palbociclib From Plasma Using an Automated and Standardized Approach for Oasis™ HLB SPE Extraction
Ce document est une note d’application et ne contient pas de section détaillée concernant l’expérimentation.
Abstract
Development and optimization of robust methods intended for use in bioanalytical laboratories is critical to ensure sensitive and reproducible assay performance. Solid phase extraction (SPE) is a common bioanalytical sample extraction technique, facilitating the isolation of the drug target from the biomatrices. SPE extraction is often perceived as complex, with many steps to optimize, often leading long method development times. This application note highlights the automated 20 Bottle reversed phase SPE optimization strategy for fast and simple SPE method development, achieving >90% analyte recovery from plasma. This approach was successful used for the bioanalytical quantification of the therapeutics drugs, sitagliptin, and palbociclib, followed by LC-MS quantification.
Introduction
Bioanalysis is a key component in drug discovery and development. It also plays an important role in determining the bioequivalence for generic drugs. Routine assays performed in these laboratories need to be sensitive, robust, and reproducible. The method development and optimization for these assays also need to be simple and standardized. SPE is often used to extract analytes of interest from complex matrices as it generally improves the selectivity, sensitivity, and robustness of the assay. Another key element for bioanalytical assays is reproducibility. Increasingly, automation is being deployed in bioanalytical laboratories to reduce inter and intra-day variability as well as to allow scientists to focus on challenging scientific questions, experimental design, and data analysis.
Reversed-Phased SPE is a compelling choice for extraction of small molecules from complex biological matrices, affording high recovery for a broad diversity of analytes. Selectivity of the sorbent bed can be optimized for the analyte of interest by finetuning the sample pre-treatment, wash, and elution solvent compositions. The Andrew+™ Pipetting Robot configured with the Extraction+ connected device allows for a completely automated, handsfree creation of calibration curve and QC samples as well as SPE execution using pre-existing OneLab™ library methods. Here, we have performed bioanalytical quantification for sitagliptin and palbociclib therapeutics as model analytes. Sitagliptin is used in the treatment of type 2 diabetes, either alone or in combination with metformin. Palbociclib is a selective CDK4 and CDK6 inhibitor used in the treatment of HR positive and HER2 negative breast cancer.
Experimental
Sample Preparation
Stock solutions (1 mg/mL) of sitagliptin and palbociclib, obtained from Sigma Aldrich (St. Louis, MO, USA), were prepared in in 100% methanol (Sigma Aldrich, St. Louis, MO, USA). This stock was serially diluted with a 5% aqueous methanol solution to prepare a 10 µg/mL working stock solution. This 10 µg/mL working stock solution was used to prepare calibration and quality control (QC) samples in rat plasma (BioIVT, Westbury, NY, USA). The calibration samples, between 1-1000 ng/mL, were prepared in duplicate, while low, mid, and high QC levels were prepared in duplicate at final concentrations of 7.5 ng/mL, 75 ng/mL, and 750 ng/mL, respectively. The Andrew+ Pipetting Robot was used to create the calibration curve and QC points, by downloading the Simple Serial Dilution method from the OneLab library (Simple Serial Dilution – OneLab (andrewalliance.com)).
SPE Extraction
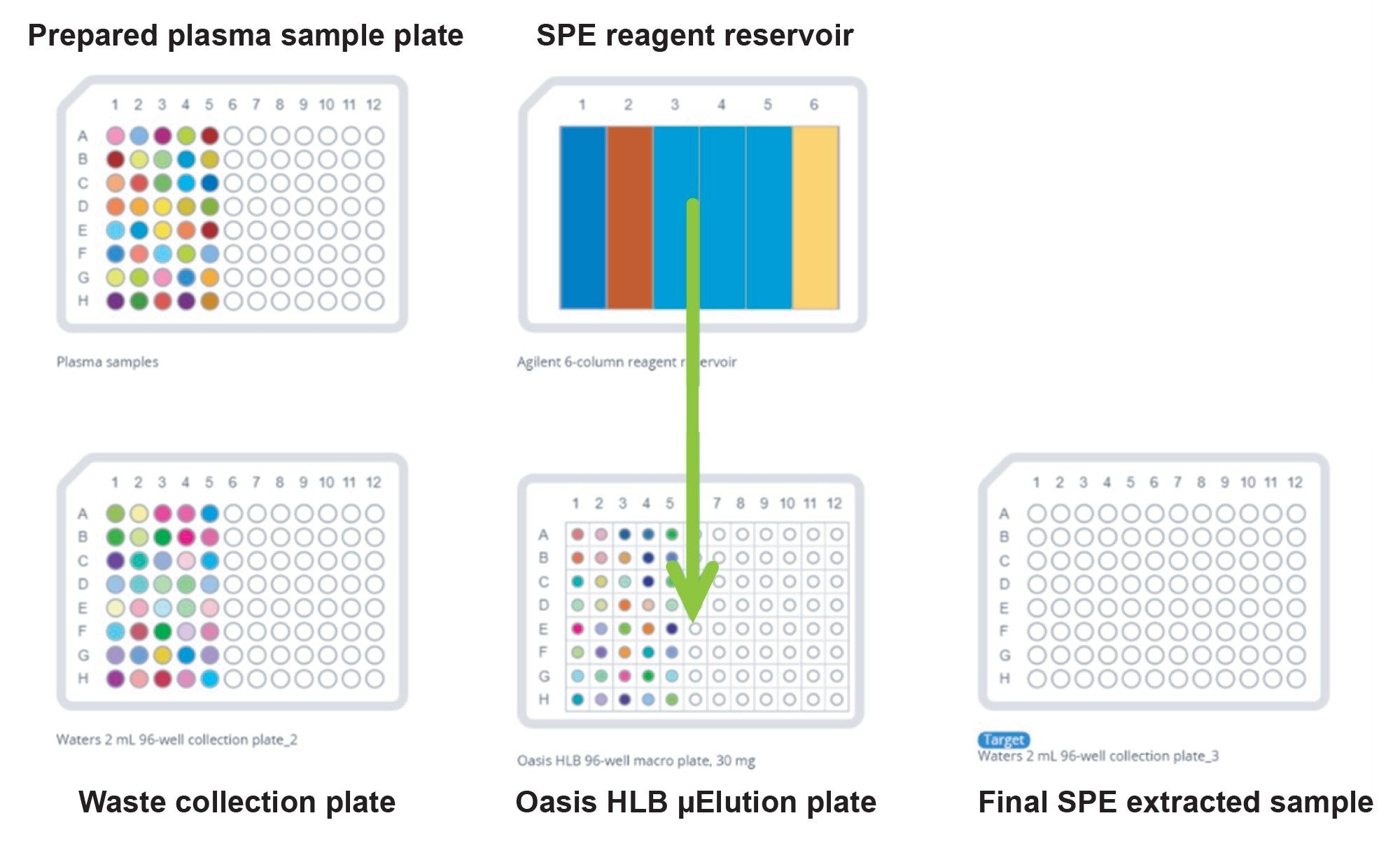
The 20 bottle Oasis HLB SPE optimization strategy , described in the care & use manual (715000109) , and previously described in application note 720008001, was used to quickly assess best SPE wash and elution conditions for sitagliptin and palbociclib. The final SPE protocol demonstrated recoveries of >90% for both sitagliptin and palbociclib and is shown in Figure 1. All extraction steps were fully automated by the Andrew+ Pipetting Robot , using the standard OneLab method downloaded from the OneLab Library (Automated Bioanalytical SPE - OneLab (andrewalliance.com). Extracted samples were injected onto a UPLC-MS/MS system.

LC-MS Analysis
Chromatographic separation for sitagliptin and palbociclib was performed using an ACQUITY™ I-Class UPLC™ and ACQUITY UPLC HSS T3 C18 Column (100 Å 2.1 x 50 mm, 1.7 µm) using a generic gradient of 5–95% B (0.1% formic acid in Acetonitrile) over 3.5 minutes. Detection was performed with a Xevo™ TQ-XS Mass Spectrometer (ESI+) using Multi Reaction Monitoring (MRM) of individual analytes. MRM transitions use for quantification of sitagliptin and palbociclib were 408.3→174.6 and 448.2→80.5, respectively.
Results and Discussion
The Andrew+ Pipetting Robot configured with Extraction+ connected device, with existing OneLab library methods were used to prepare samples and execute the 20 Bottle SPE optimization and final Oasis HLB SPE plasma quantification experiments. For this work, the Simple Serial Dilution method was downloaded from the OneLab library to easily prepare the calibration curve and QC plasma samples. SPE was performed on the automation platform by downloading Automated Bioanalytical SPE method (Figure 2).
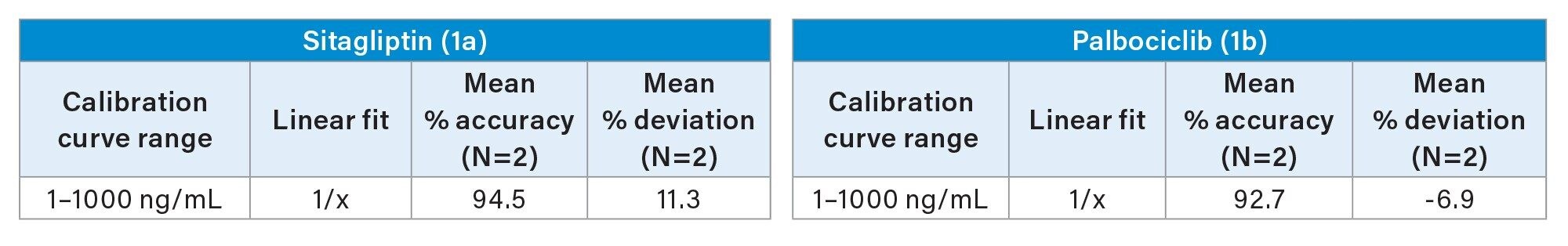
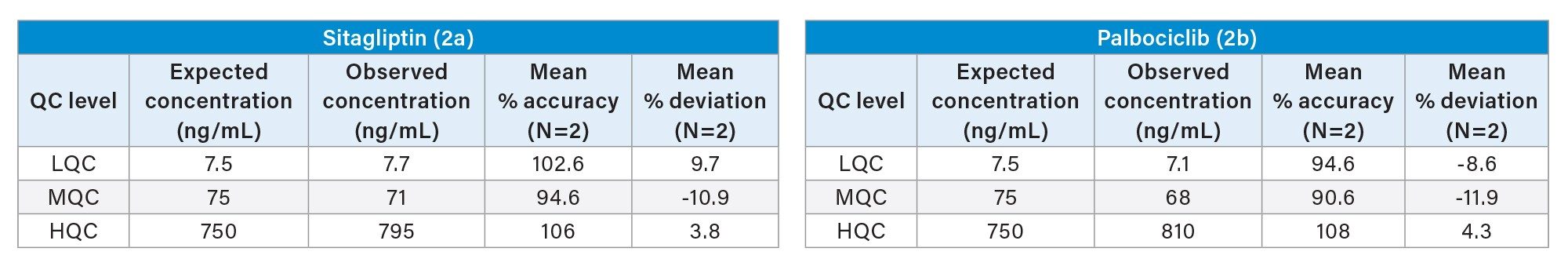
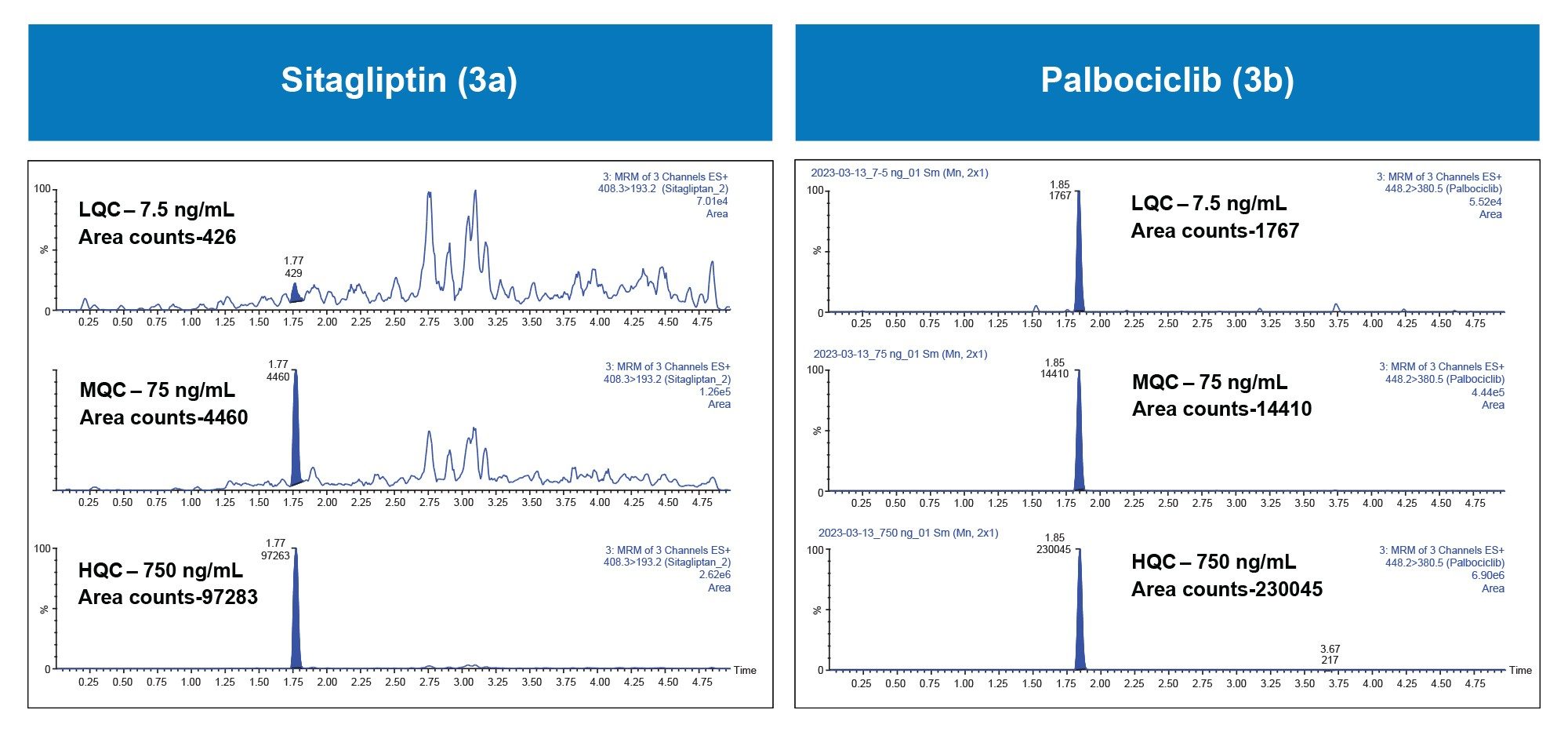
Quantification performance for sitagliptin and palbociclib was excellent with linear dynamic range (>0.99) from 1–1000 ng/mL using 1/x regression, with mean accuracies of 92.7 and 94.5%, respectively. These results are highlighted in Table 1. All QC levels for sitagliptin and palbociclib passed the bioanalytical method validation criteria of percent accuracy and percent deviation of +/-15%. The mean accuracies for QC levels ranged from 90.6–108 % and mean deviation values ranging from -11.9–9.7 % (Table 2a and 2b). Representative QC chromatograms demonstrated a linear increase in MS area counts with increase in analyte concentration for sitagliptin and palbociclib. This performance is highlighted in Figure 3, panel a and b, respectively.
Conclusion
Developing and optimizing SPE sample extraction and LC-MS/MS methods for use in bioanalytical laboratories is perceived to be challenging, with complex protocols and requiring long method development. This work highlights a two experiment fully-automated approach for fast reversed-phase Oasis HLB SPE method optimization, to achieve high analyte recovery with excellent accuracy and reproducibility from plasma using the Andrew+ Pipetting Robot configured with Extraction+ connected device. Quantitative performance of sitagliptin and palbociclib extracted from rat plasma was excellent, with linear dynamic ranges from 1–1000 ng/mL, QC accuracies between 90.6–108%, and RSDs between -11.9–9.7%, which meet recommended small molecule LC-MS method validation guidance. The combination of generic protocols and automated sample preparation greatly simplifies and streamlines sample extraction, maximizing lab productivity, reducing errors, and ensuring overall bioanalytical method performance.
720007996, November 2023