Demonstrating the Applicability of the ACQUITY™ Premier Binary System for Long Shallow Gradient Peptide Mapping Analysis
Abstract
Reversed-Phase Liquid Chromatography (RPLC) is a key analytical tool in peptide mapping analysis. Complex peptide maps may require long shallow gradients to adequately resolve the multitude of digested product and product variant peptides. The ACQUITY Premier Binary System with MaxPeak™ HPS Technology is ideally suited for this task given its high gradient fidelity. When compared to a competitive Biocompatible UHPLC System over the course of a five day study, the ACQUITY Premier System demonstrated superior retention time repeatability.
Benefits
- Superior retention time repeatability based on high shallow-gradient fidelity at meaningful flow rates for ultraviolet (UV) and mass spectrometry (MS) based detection
- Simplified peptide map comparability without the need for peak alignment algorithms
Introduction
RPLC is a widely used chromatographic method in peptide mapping analysis. To achieve a detailed picture of complex biomolecules such as monoclonal antibodies (mAbs), a variety of digestion enzymes may be used to cleave the protein at various points, resulting in a mixture of smaller peptides which can then be analyzed for greater understanding of the molecule. Reversed-phase ultra-high performance liquid chromatography (RP-UHPLC) is the chromatographic method most often employed in the modern analysis of peptide maps of biotherapeutic proteins.1
Waters' ACQUITY Premier System (Figure 1) equipped with a binary pump solvent delivery module is well suited for this type of analysis. The high-pressure mixing binary pump is ideal for peptide mapping applications, specifically those with shallow gradients, which require precise composition reproducibility in order to achieve repeatable peak retention times. In this application note, an enolase digestion was analyzed over five days on an ACQUITY Premier System and a competitive biocompatible system using a common column and sample. The results were compared between systems by monitoring retention times of peptides spread throughout the gradient profile. Overall, both systems performed well, however, the ACQUITY Premier System showed superior retention time repeatability compared to the competitor system.

Experimental
Sample Description
Two vials of MassPREP Enolase Digestion Standard (p/n: 186002325) were reconstituted in 100 µL of 0.1% trifluoroacetic acid (TFA) in water. Both vials were vortexed for ten seconds to ensure reconstitution and pooled together. Pooled samples were vortexed for ten seconds and 90 µL was transferred into two Total Recovery Vials (p/n: 186002805) which were immediately place in a chilled autosampler.
LC Conditions
|
LC system: |
ACQUITY Premier Binary System with ACQUITY APC™ Reservoir Cap Kit (p/n: 205001152), Competitor X Biocompatible UHPLC Binary System with standard reservoir caps |
|
Detection: |
Photodiode Array Detector (PDA) (ACQUITY Premier System), Diode array detector (DAD) (Competitor X), 214 nm @ 10 Hz |
|
Vials: |
Total Recovery (p/n: 186002805) |
|
Column(s): |
ACQUITY UPLC™ Peptide CSH™ C18, 130 Å, 1.7 µm, 2.1 x 150 mm (p/n: 186006938) |
|
Column temp.: |
65 °C |
|
Sample temp.: |
6 °C |
|
Injection volume: |
10 µL |
|
Mobile phase A: |
0.1% TFA in water |
|
Mobile phase B: |
0.1% TFA in acetonitrile |
|
Needle wash/purge solvent: |
25/75 Mobile phase A/Mobile phase B |
|
Seal Wash: |
80/20 water/methanol |
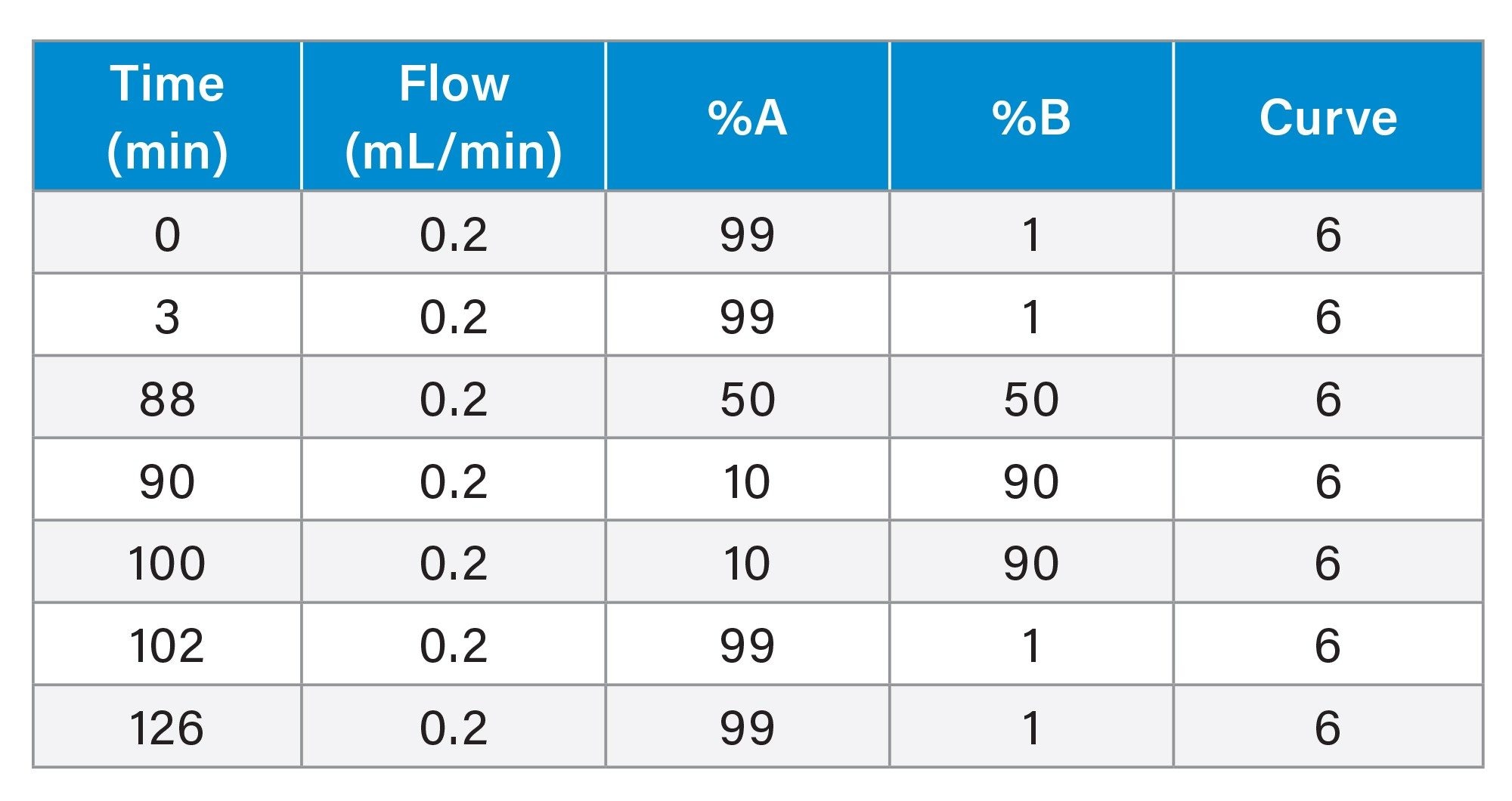
Gradient Table
Data Management
|
Chromatography software: |
Empower™ 3.6.1 |
Results and Discussion
Solutions containing peptides created by enzymatic digestion of biomolecules are often complex, containing tens to hundreds of peptide peaks varying widely in biophysical properties. In general, hydrophobic properties of a peptide are driven by the length of the peptide or increasing number of alkyl and aromatic amino acid residues in the peptide fragment.2 The resulting mixture leads to complex chromatograms, or peptide maps, that need to be carefully analyzed to understand what peak corresponds to a peptide in order to understand the molecule. In order to resolve the many peaks in these maps, long shallow reversed-phase gradients are often employed where the percent organic in the mobile phase is slowly increased.3 The small changes in organic content over a long gradient allow for resolution of peptides with similar hydrophobicity. The ability of the pump on the chromatographic system to precisely deliver these small changes in organic composition is critical to the repeatability of results. Any small changes in organic composition throughout the gradient profile will result in shifting peak retention times, making data interpretation and comparison difficult.
The ACQUITY Premier Binary System is ideally suited for peptide mapping separations as the high-pressure mixing binary pump offers superb solvent delivery precision. In this application note, the ACQUITY Premier Binary System is compared to a leading competitor’s Biocompatible UHPLC Binary System (Competitor X) using retention time repeatability as a measure of pump performance.
To assess the repeatability of the systems, five days of testing were performed over the course of two weeks on the ACQUITY Premier and the competitive system using a method adapted from previous work.4 Mobile phases and samples were prepared daily, and systems were run simultaneously with a new column provided at the start of testing for each system. Low evaporation bottle caps were used to ensure solvent stability due to the long sample set times and volatility of mobile phase components. Sample sets included six replicate injections of Waters Enolase Digestion Standard interspersed with blank mobile phase injections. Systems were qualified for performance prior to the study. Before the start of each sample set, a dynamic leak test was performed to ensure the pumps were working correctly and no leaks were present on the system that might affect gradient delivery precision.
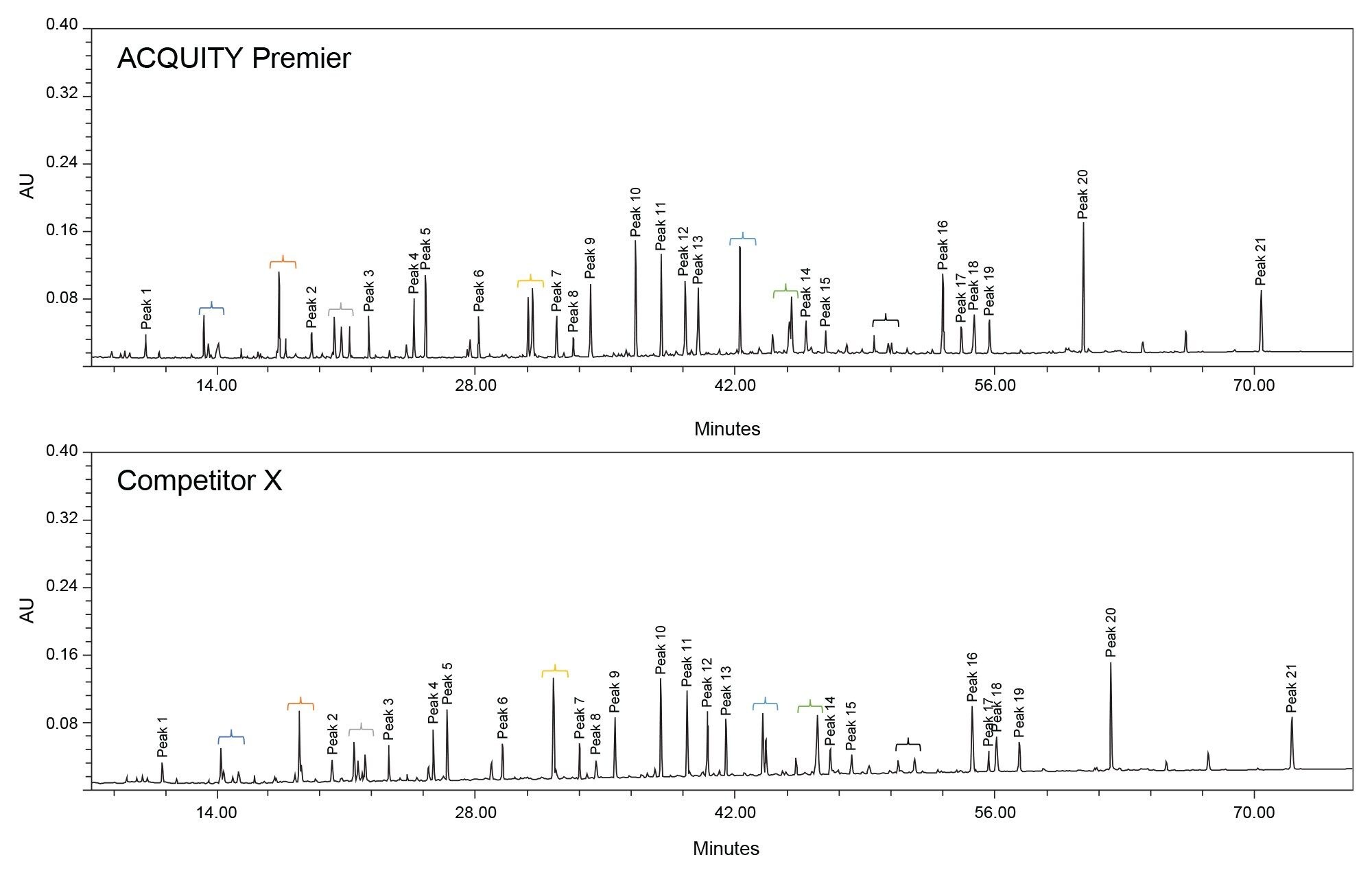
Representative chromatograms of the Enolase Digestion Standard on both systems are displayed in Figure 2. For this analysis, organic content in the mobile phase starts very low (1%) in order to fully resolve the early eluting highly polar peptides contained in the digest. A long, shallow gradient was employed with a mobile phase organic change of 0.58 %/min over 85 minutes in order to achieve resolution for most analytes, as well as test the solvent delivery precision of the binary pumps. Absolute retention times on the competitive system are slightly higher than that of the ACQUITY Premier System due to increased system volume caused by a column switching valve in the column compartment as well as different gradient delay volumes. Additionally, minor changes in selectivity are apparent between the two systems and are highlighted with brackets in the figure. As described earlier, the complex nature of the sample results in many structurally similar peptides, which may be difficult to separate chromatographically. Thus, any slight changes in gradient delivery, column temperature control, solvent mixing, and/or gradient delay volume could lead to detectable changes in selectivity. This is a testament to the difficulty in transferring established methods between systems, even those specifically designed to limit system-analyte interactions.
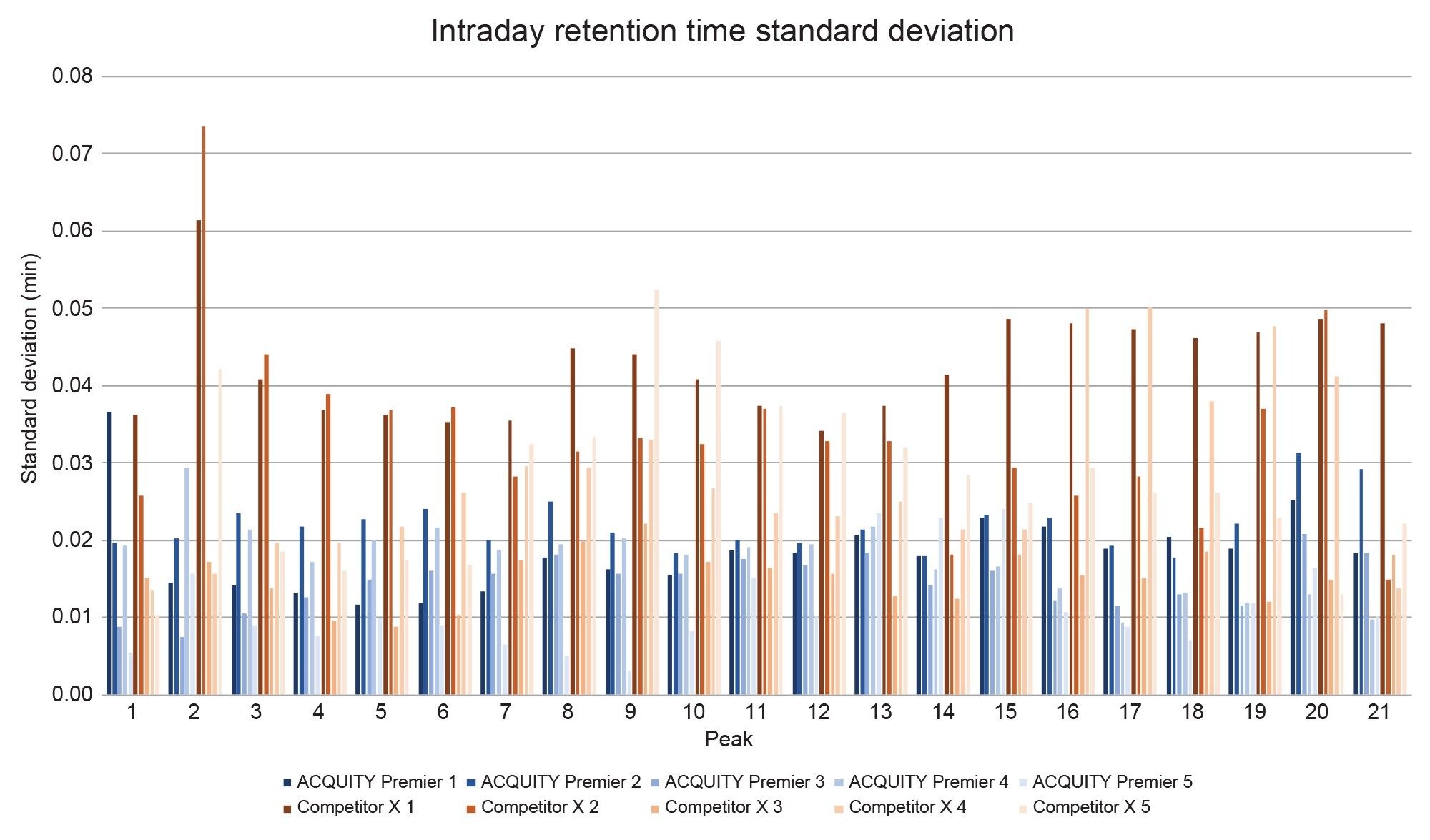
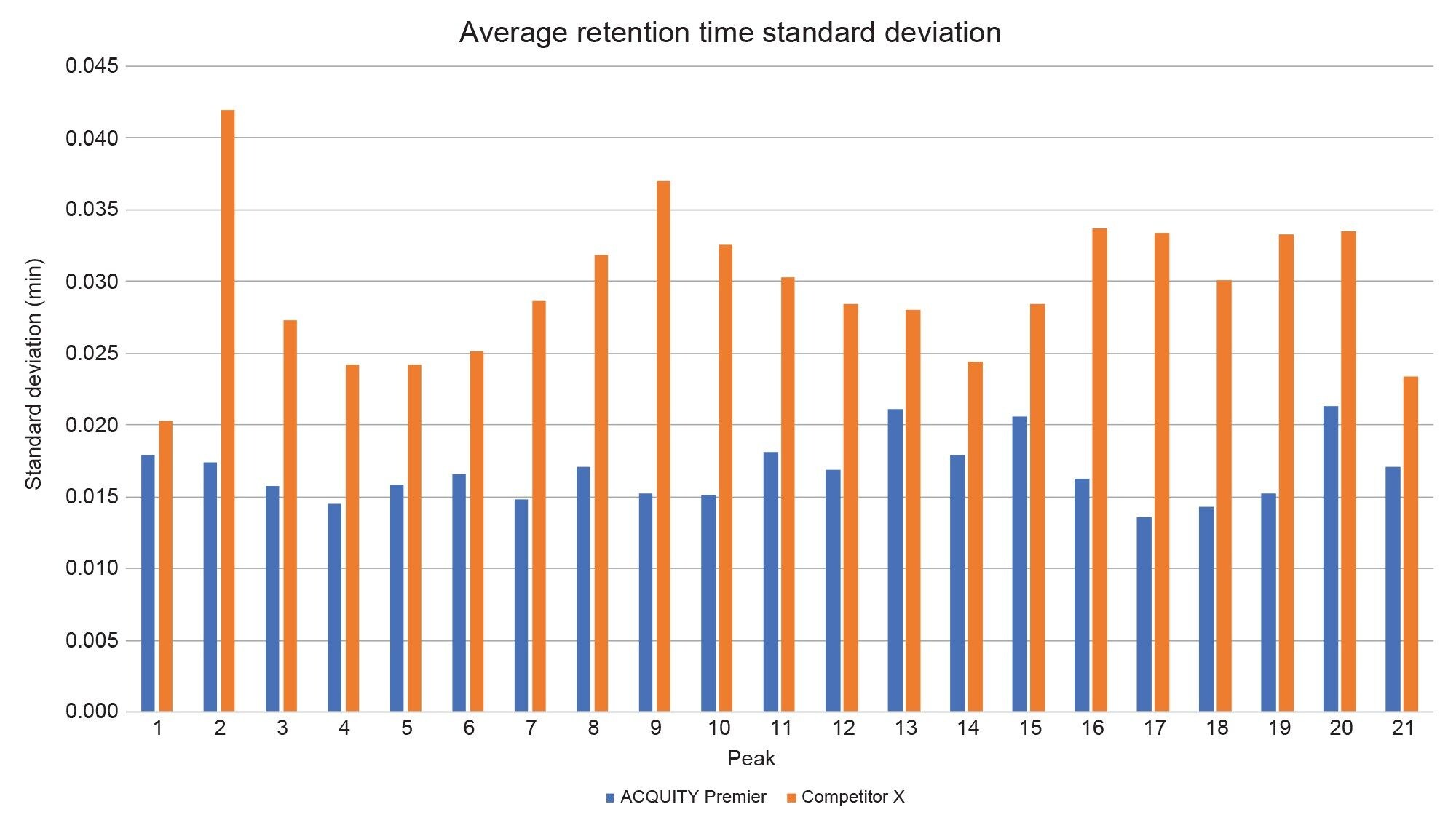
To assess each system’s performance, 21 peptide peaks were monitored for retention time repeatability within sample sets and across five days of testing. Care was taken to exclude co-eluting peaks and those that experienced changes in selectivity between systems. Figure 3 shows the daily retention time standard deviation averages for all 21 peaks on both systems. No discernable trend is observed across the days within a system set. Figure 4 displays the total standard deviation average across all five days of testing. Differences in mobile phase preparation, sample preparation, room temperature, column temperature, and autosampler temperature can all lead to slight variations in absolute retention time which are not indicative of pump performance. The average standard deviation across days was calculated by taking the mean of the intraday standard deviation to eliminate these outside sources of variation. In all cases the ACQUITY Premier System displayed lower retention time standard deviation with improvements over the competitive system ranging from 13% (Peak 1) to 146% (Peak 17). This indicates that the binary pump on the ACQUITY Premier System was able to deliver a more reproducible and repeatable gradient over several injections and across multiple days. Overall, the binary pumps on both systems performed to specification, with the ACQUITY Premier System achieving an average standard deviation across all peaks of 0.017 minutes while the competitive system achieved 0.030 minutes, with all standard deviation averages falling below 0.045 minutes (Figure 4).
Conclusion
Reversed-phase peptide mapping analysis is an important tool used for characterization and attribute-based analysis of biotherapeutics. Chromatographic systems with binary pumps are ideally suited for these separations as they deliver more precise gradients when compared with quaternary pumping systems. In this work, the ACQUITY Premier Binary System was compared to a competitive UHPLC binary system for their ability to deliver long shallow gradients typical in peptide mapping studies. Overall, both systems performed well, with all 21 peaks monitored throughout the peptide map of an Enolase Digestion Standard achieving a fiveday average standard deviation no-greater than 0.045 minutes. However, the ACQUITY Premier System outperformed the competitive system for all 21 peaks, with improvements in average standard deviations ranging between 13% and 146%. This study demonstrates the applicability of the ACQUITY Premier Binary System for peptide mapping studies, specifically those employing long shallow gradients which can be challenging for a pump to deliver reproducibly.
References
- Zhu, R.; Zacharias, L.; Wooding, K. M.; Peng, W.; Mechref, Y. Chapter Twenty-One - Glycoprotein Enrichment Analytical Techniques: Advantages and Disadvantages. In Proteomics in Biology, Part A; Shukla, A. K., Ed.; Methods in Enzymology; Academic Press, 2017; Vol. 585, pp 397–429. https://doi.org/10.1016/bs.mie.2016.11.009.
- Mitulovic, G.; Mechtler, K. HPLC Techniques for Proteomics Analysis - a Short Overview of Latest Developments. Briefings in Functional Genomics 2006, 5 (4), 249–260. https://doi.org/10.1093.
- Gruber, K. A.; Stein, S.; Brink, L.; Radhakrishnan, A.; Udenfriend, S. Fluorometric Assay of Vasopressin and Oxytocin: A General Approach to the Assay of Peptides in Tissues. Proceedings of the National Academy of Sciences 1976, 73 (4), 1314–1318.
- Simeone, J.; Hong, P.; McConville, P. R. Performance of the ACQUITY UPLC I-Class PLUS System for Methods Which Employ Long, Shallow Gradients. Waters Application Note, 720006290, 2018.
720007631, June 2022