Transferring methods between internal laboratories and external contract organizations is a key part of analytical development of biopharmaceuticals. The sending and receiving laboratories are faced with the challenges of each laboratory updating instrumentation on their own respective timelines as older instrumentation is phased out, as well as each laboratory having instrumentation from different vendors. Regardless of these differences, any data generated between the two laboratories must be demonstrated to be comparable. Although development and quality control laboratories may be slow to replace existing technologies, modern technologies can benefit legacy methods and future development activities as part of lifecycle management. The Arc Premier System was introduced as a modern UHPLC that can also support more routine assays traditionally run on HPLC platforms. Size exclusion and ion-exchange chromatography methods, two assays commonly used in quality control, showed reproducible results when migrating methods from an Alliance HPLC System. Differences in retention time and peak area percent were negligible between the two platforms, instilling confidence that the legacy method could be replicated successfully on the Arc Premier System. To further take advantage of the more modern UHPLC technology, column chemistries were also updated to achieve results that could be more reliably quantitated through improved chromatographic performance.
Because the lifecycle of biopharmaceutical products spans many years, it is critical that the analytical methods used to ensure product quality are robust and reliable. These methods further require that the instrument platforms and column chemistries used for analysis can deliver consistent results. In the biopharmaceutical space, size exclusion chromatography (SEC) and ion-exchange chromatography (IEX) are among the most common assays used to assess product quality of monoclonal antibodies (mAbs). As legacy methods are transferred between both internal and external laboratories, it is critical that these methods can be replicated, regardless of environmental factors that may differ between the sending and receiving laboratories.
In this work, SEC and IEX methods are migrated from an Alliance HPLC System, an industry standard stainless-steel HPLC system, to an Arc Premier System, a modern UHPLC platform. The Arc Premier System is designed with MaxPeak High Performance Surfaces (HPS) Technology, which was specifically developed to mitigate unwanted adsorption of metal-sensitive analytes to metal surfaces.1 Although this technology has demonstrated benefits for RPLC and HILIC assays due to the intrinsic charge properties of the analyte and metal surface which are generally not observed in SEC and IEX due to the high-ionic strength of the mobile phases, it is important that new technologies can be more broadly deployed and support existing methods. After demonstrating successful method migration using traditional SEC and IEX methods, updated column chemistries are used in combination with the Arc Premier System to show that modern technologies can both support legacy methods as well as offer distinct advantages over these more conventional approaches.
Formulated trastuzumab was prepared to 10 mg/mL in mobile phase per USP <129> for SEC experiments. Trastuzumab was diluted in water to 1 mg/mL for IEX experiments. Sample was prepared fresh prior to each analysis.
|
LC systems: |
Alliance HPLC System with 2489 UV/Visible Detector Arc Premier System with 2489 UV/Visible Detector |
|
SEC |
|
|
Columns: |
BioSuite Diol (OH) Column 250 Å, 5 µm, 7.8 x 300 mm, USP Classification L59 (p/n: 186002165) BioResolve SEC mAb Column, 200 Å, 2.5 µm, 7.8 x 300 mm (p/n: 186009441) |
|
Wavelength: |
280 nm |
|
Injection volume: |
20 µL |
|
Column temp.: |
Ambient |
|
Flow rate: |
0.500 mL/min |
|
Mobile phase: |
200 mM potassium phosphate buffer, 250 mM KCl, pH 6.2 |
|
Method: |
30 min isocratic run time |
|
Columns: |
Protein-Pak Hi Res CM Column, 7 µm, 4.6 x 100 mm (p/n: 186004929) Protein-Pak Hi Res SP Column, 7 µm, 4.6 x 100 mm (p/n: 186004930) BioResolve SCX mAb Column, 3 µm, 4.6 x 100 mm (p/n: 186009060) |
|
Wavelength: |
280 nm |
|
Injection volume: |
20 µL |
|
Column temp.: |
25 °C |
|
Mobile phase A: |
20 mM MES buffer pH 6.6 |
|
Mobile phase B: |
20 mM MES buffer pH 6.6, 1 M NaCl |
Empower 3 Chromatography Data Software FR4
SEC is the method of choice for studying size variants, such as high molecular weight species (HMWS) or aggregates, in the development and quality control of mAbs. Aggregation can occur due to properties inherent to the mAb or through external factors during manufacturing and storage and has been linked to increased immunogenicity. Legacy SEC methods using columns with large particle sizes and HPLC systems with high system dispersion provide adequate resolution of the HMWS and the monomer peak. Although low molecular weight species (LMWS) are also present, capillary electrophoresis has historically been used for more reliable quantitation as HPLC-based methods provide insufficient resolution. While advances in SEC have allowed for better resolution of the LMWS, it is still important that as new LC platforms are incorporated into an evolving laboratory environment, that these systems continue to support legacy methods and generate the same results as more traditional LC platforms.
To assess a legacy SEC method, method conditions were selected according to USP <129> which provides analytical procedures for purity assessment of mAbs by SEC, capillary electrophoresis, and oligosaccharide analysis. SEC conditions are summarized in Table 1 and were used without making any changes to the proposed method. A series of six injections of trastuzumab were run on an Alliance HPLC System and an Arc Premier System. Representative chromatograms show similar profiles between the two platforms (Figure 1).
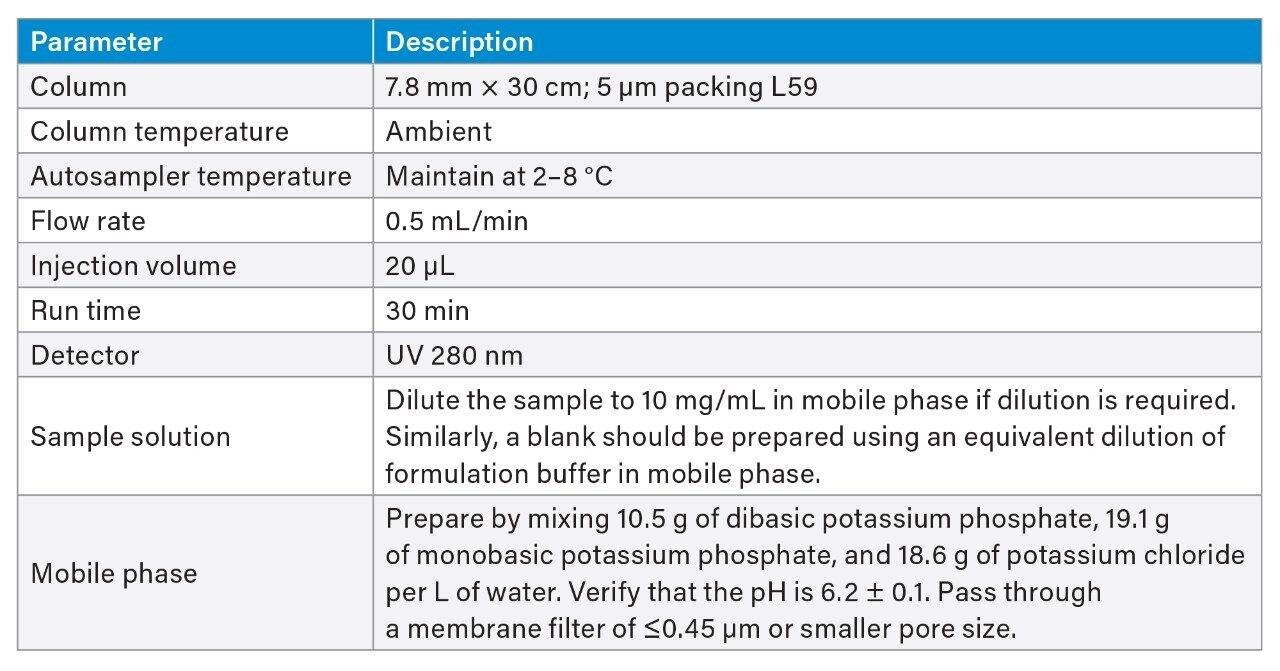
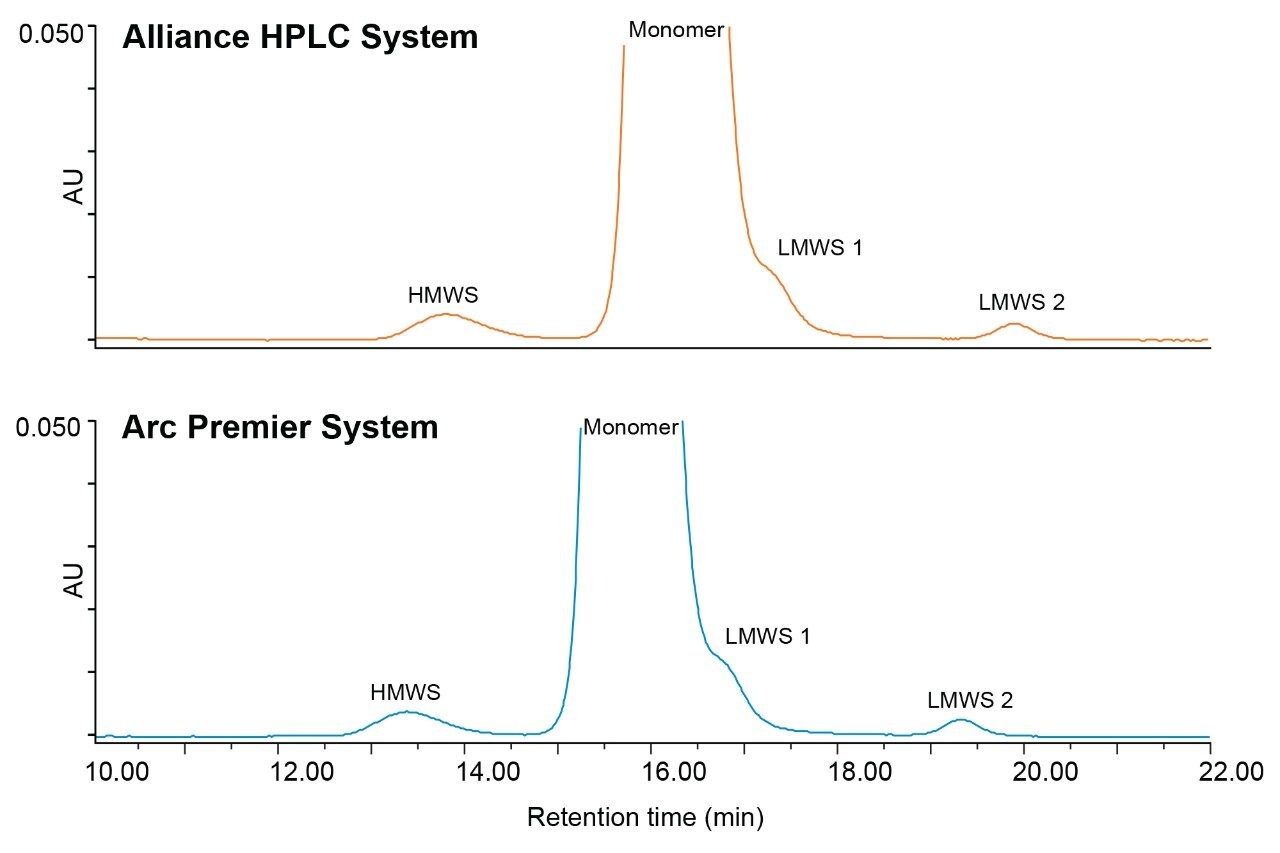
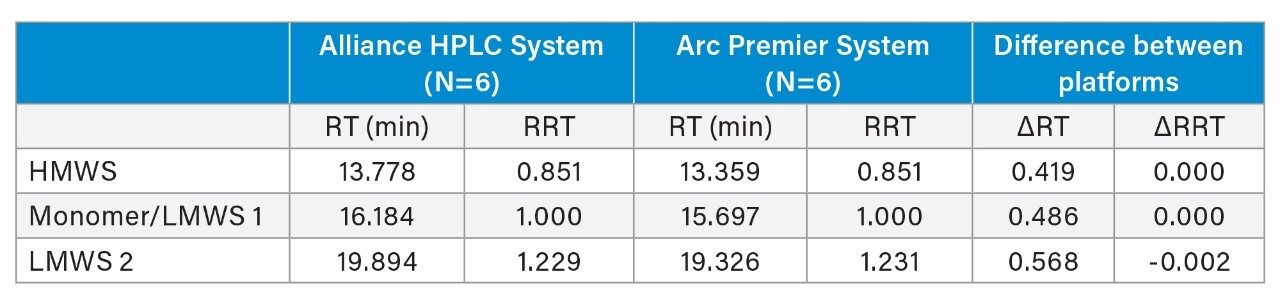
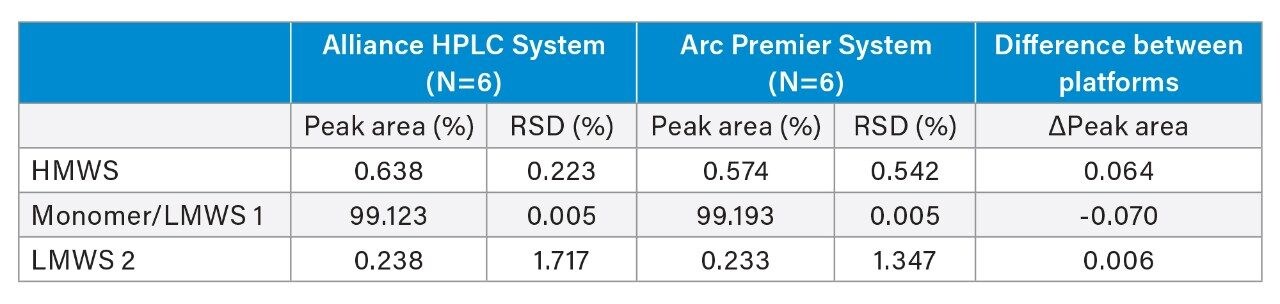
Retention time and peak area were further compared to address method equivalency. Table 2 reports the average retention time and relative retention time for six injections on each LC platform for the HMWS, monomer, and LMWS 2 (LMWS 1 co-elutes with the monomer peak). The difference in retention time between the two systems is less than 0.6 minutes for each of the peaks. To account for shifts in retention time, the difference in relative retention time is also reported, and is negligible. Peak area was also conserved between platforms (Table 3). The difference in peak area between platforms was no greater than 0.07% for six injections, which was reported for the monomer peak. The HMWS and LMWS 2 which are far less abundant, were also reliably quantitated with differences in peak area of 0.064% and 0.006%, respectively. Furthermore, results obtained on a single LC platform were also repeatable. The %RSD did not exceed 2% for any of the peaks on either platform. The combination of retention time and peak area show that the Arc Premier System yields results that align with a legacy LC system.
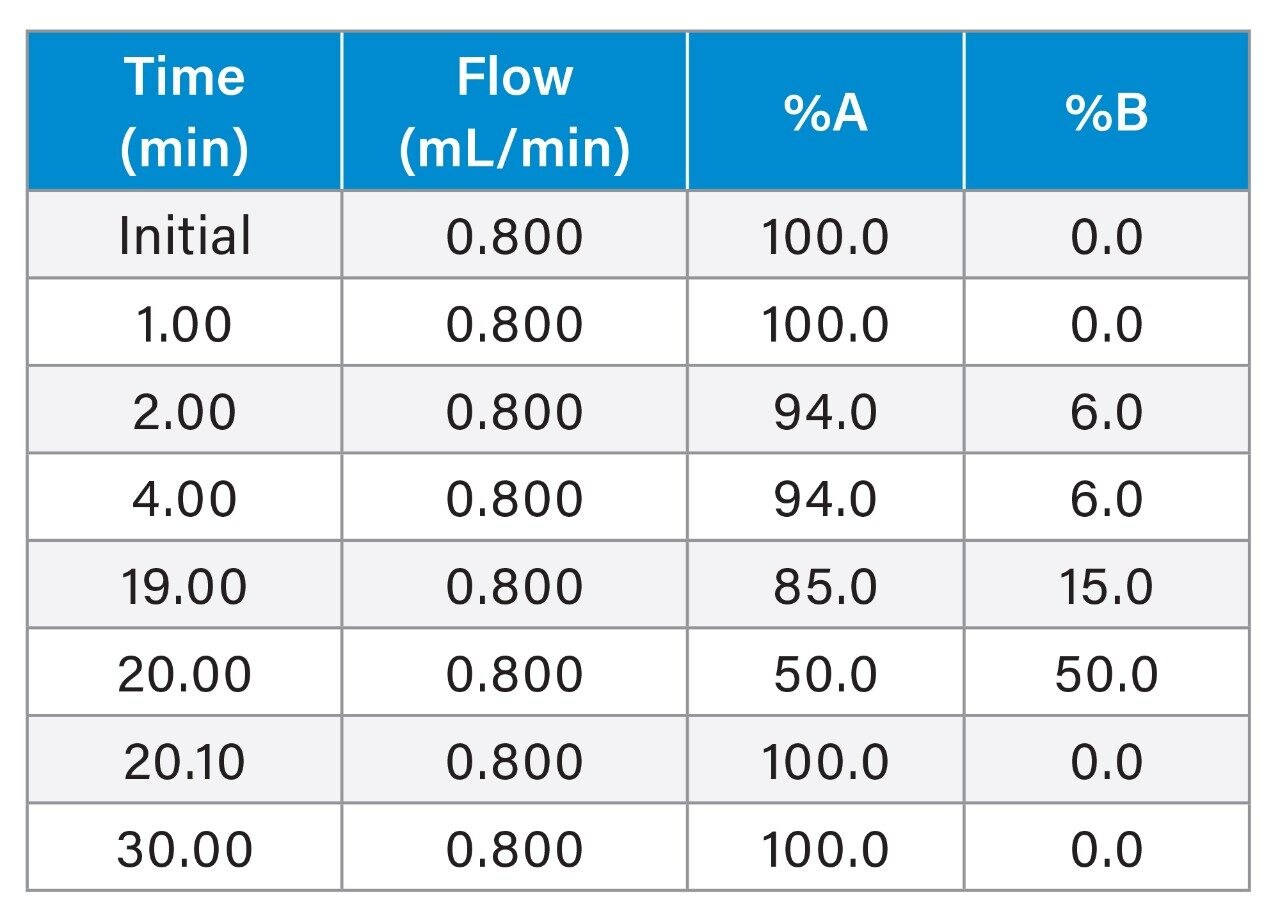
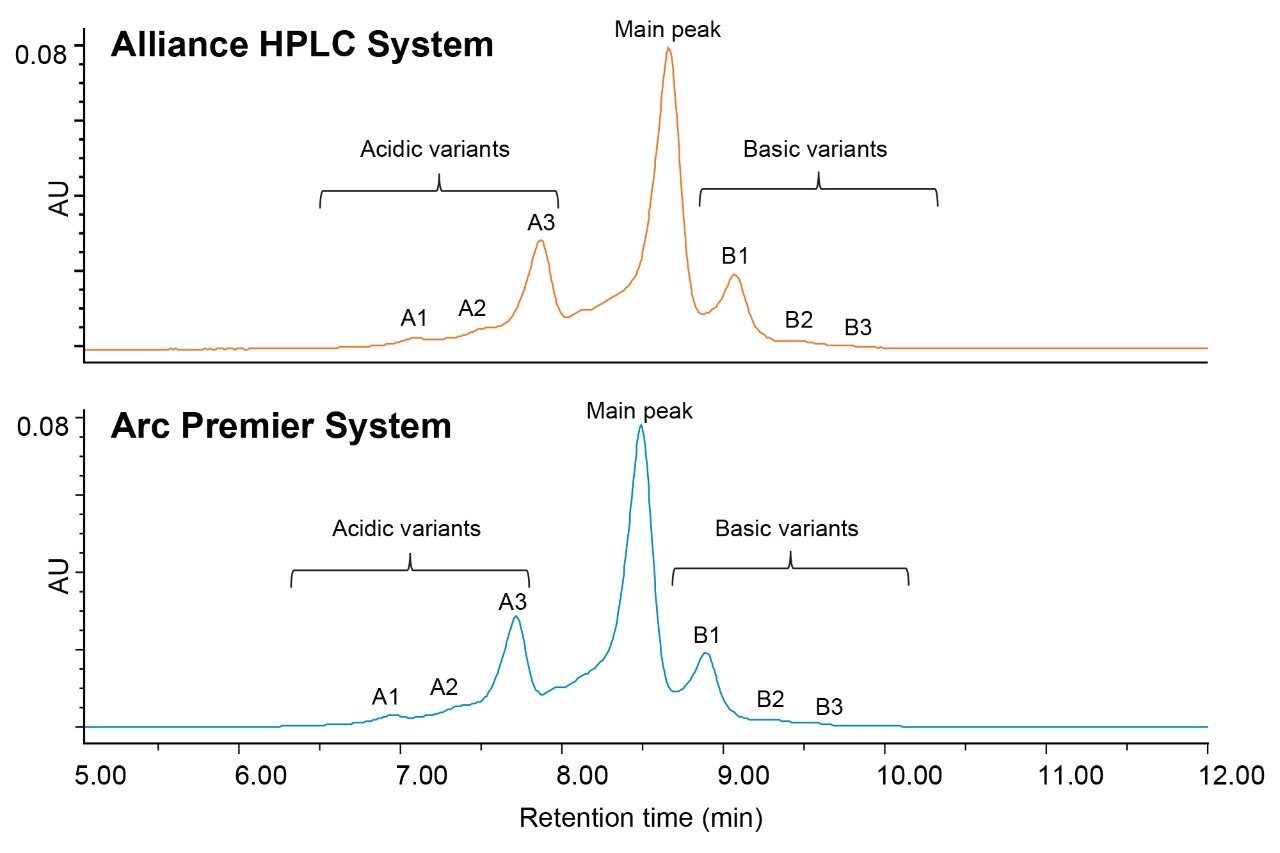
To test the broader applicability of the Arc Premier System, IEX was also evaluated as a routine assay performed in QA/QC environments. IEX is a charge-based separation used for quantitation of acidic and basic impurities where traditional methods most frequently use a salt gradient (versus a pH gradient) for elution. Because IEX methods need to be optimized for the specific analyte, guidance documents offering more generalized method recommendations are not available. IEX method conditions were first optimized using a Protein-Pak Hi Res CM Column (7 µm, 4.6 x 100 mm) on the Alliance HPLC System to represent a method using more traditional LC and column technologies. Six injections of 1 mg/mL trastuzumab were run on the Alliance HPLC System and the Arc Premier System for comparison. Chromatographic profiles were observed to be highly similar between the two platforms (Figure 2). As expected, a shift in retention time is observed due to systematic differences in dwell volume (Alliance HPLC System: 1050 µL; Arc Premier System: 950 µL). Although legacy methods often use long gradient/run times, a shorter method was used because chromatographic profiles and assignment of acidic and basic variants match historical results obtained internally.2,3
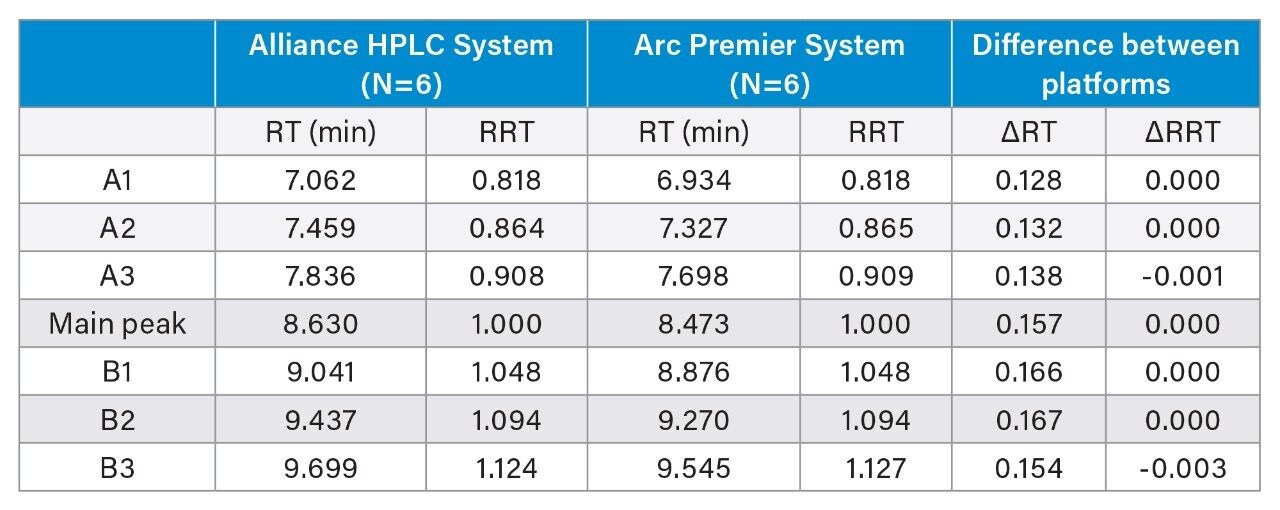
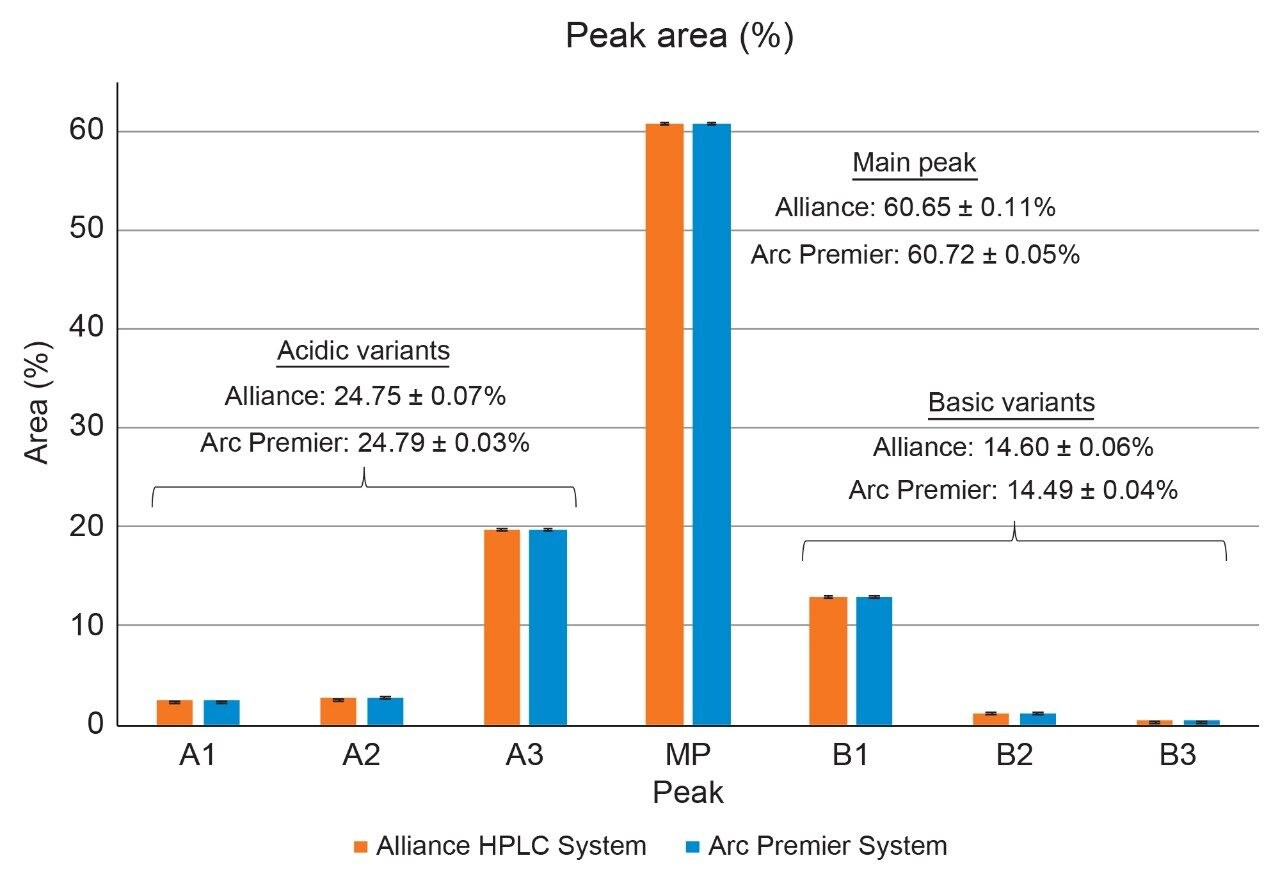
To further evaluate the results between LC platforms, Table 4 reports the retention time and relative retention time of the seven peaks identified in Figure 2. The average difference in retention time is 0.149 minutes, which aligns with the difference in dwell volume between the two platforms (0.149 minutes x 0.8 mL/min = 0.126 mL). When correcting for this difference through reporting relative retention time, the difference is negligible. Relative peak area for these same seven peaks is also highly similar, where individually reported peaks are within error of one another (Figure 3). When reporting the total acidic and basic variants, results between the two platforms were within 0.04 and 0.11 percent of one another, respectively. The Alliance HPLC System and the Arc Premier System independently produced repeatable data while also demonstrating that comparable results were obtained when comparing the two platforms.
By replacing legacy LC platforms and introducing more modern instrumentation into the laboratory, new chemistries with smaller particle sizes can also be incorporated to build more robust methods, offering benefits such as increased resolution and faster run times. In Figure 4A, SEC data was collected for trastuzumab using method conditions from USP <129> where the recommended column was updated to a column with smaller particle (and pore) size. In the legacy method, LMWS 1 is generally not accurately quantitated as it is similar in size to the monomer and elutes as a back shoulder. By updating the column chemistry to the BioResolve SEC mAb Column, this back-shoulder peak can now be resolved and was determined to over-estimate the peak area percent of the monomer peak by approximately 0.6% (Figure 4A).
To see if these same benefits could be achieved in IEX, the Arc Premier System was used to evaluate 7 µm column chemistries with both strong cation-exchangers (Protein-Pak Hi Res SP Column) and weak cation-exchangers (Protein-Pak Hi Res CM Column) and the BioResolve SCX mAb column, which is a strong cation-exchange column with 3 µm particle size (Figure 4B). When using the same method conditions across all three columns, the BioResolve SCX mAb Column resolved the greatest number of variants. Furthermore, the total number of acidic and basic variants aligns with results reported for the Protein-Pak Hi Res SP Column. While the Protein-Pak Hi Res CM Column, the weak cation-exchange column, reports a similar peak area purity to the other two columns, the total number of acidic and basic variants is more variable when compared to the strong cation-exchange columns. Unlike SEC which can show resolution gains from minimizing LC system dispersion alone, IEX methods can benefit from further optimization for different column chemistries and analytes. Because in IEX there is generally a trade-off when resolving acidic and basic variants- to improve the resolution of one the resolution of the other will typically decrease, it can be beneficial to tailor methods to better balance the resolution of each impurity group.
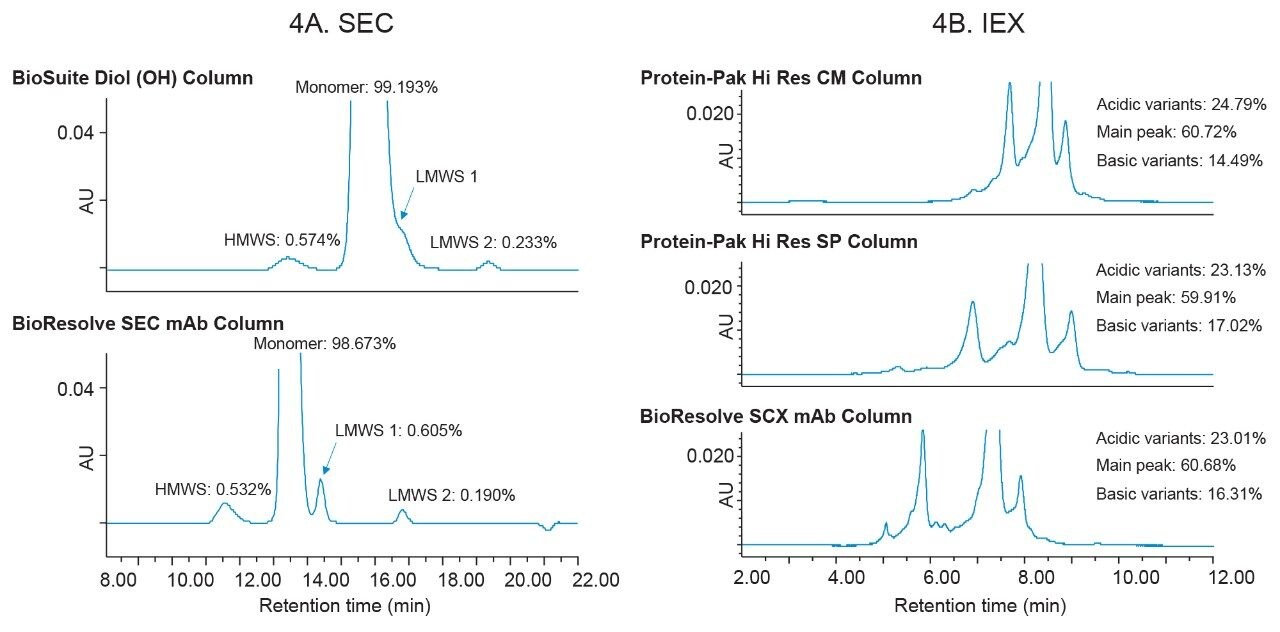
Traditional SEC and IEX methods employ HPLC systems and column chemistries having large particle size to avoid exceeding pressure limitations of these more aged platforms. As more modern LC platforms are incorporated into the laboratory, these new systems must demonstrate that they can produce comparable results to existing technologies. This work demonstrates that legacy SEC and IEX methods could be migrated from an Alliance HPLC System to an Arc Premier System with negligible differences in retention time and peak area percent. By combining the Arc Premier System with modern UHPLC columns, data could be more accurately interpreted through greater efficiency, selectivity, and enhanced resolution.
720007283, Revised August 2021