This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the improved sensitivity of the Waters SYNAPT XS HDMS System for the characterization of monoclonal antibodies in denatured and native states.
The SYNAPT XS HDMS System offers enhanced sensitivity and resolution to provide high-quality characterization data for intact biotherapeutics.
High resolution mass spectrometry is a powerful characterization tool for intact mass analysis of complex biomolecules and is, therefore, increasingly employed in bioanalytical characterization labs for the discovery and development of biopharmaceutical drugs. The boundaries of analytical sensitivity must be continually challenged, especially in discovery stage here sample amounts may be very limited. The SYNAPT XS HDMS (high definition mass spectrometer) is the newest in research-grade, high-resolution QToF (quadrupole time-of-flight) mass spectrometers, offering enhanced capabilities and technologies to address these challenges. It is equipped with StepWave XS technology for improved ion transmission and sensitivity, as well as an extended flight tube for better resolution. These enhanced performance characteristics have been compared in previously published experiments.1,2 In this application brief, we demonstrate the advanced sensitivity and resolution of the SYNAPT XS with a dilution series of trastuzumab in both denaturing and native states.

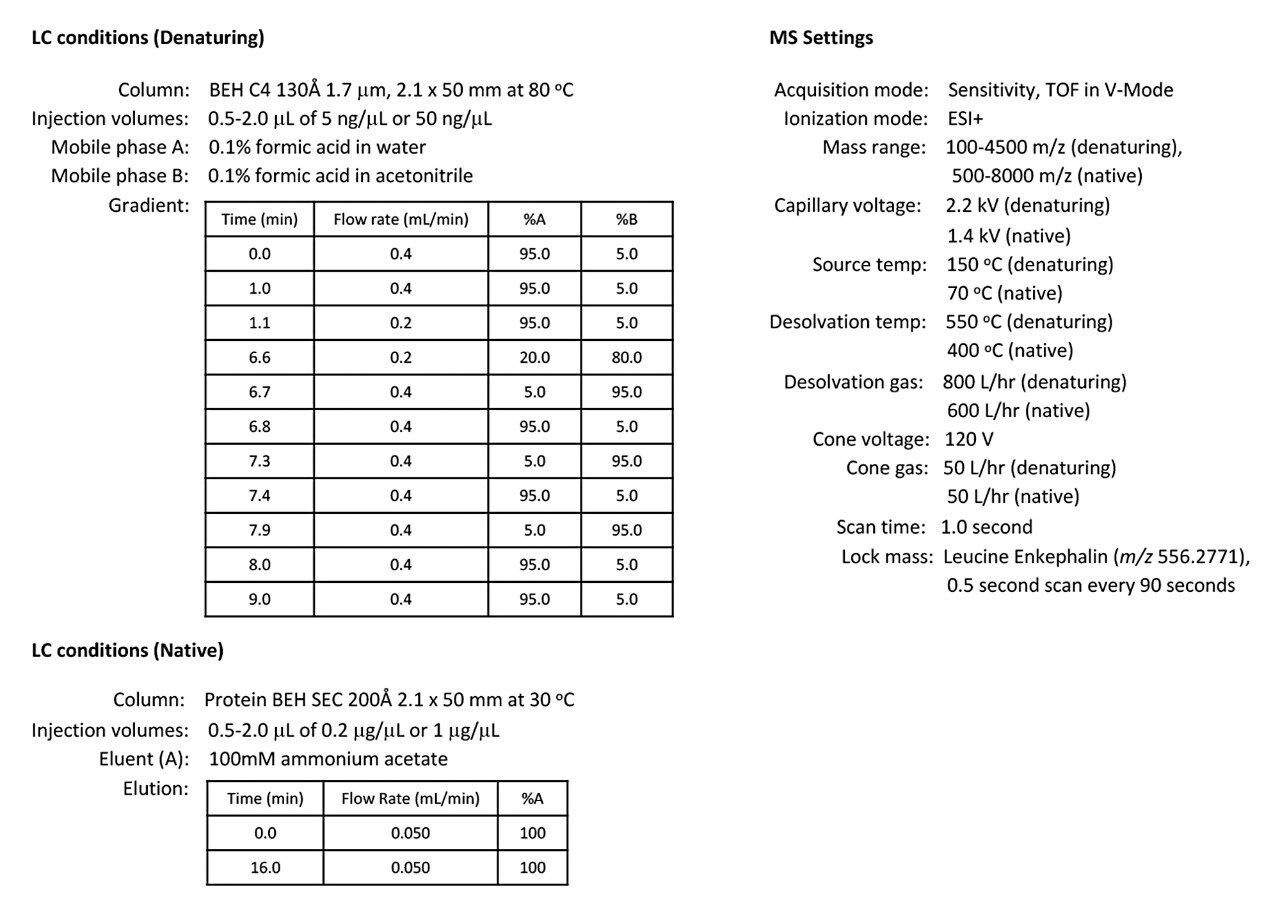
To explore the sensitivity of the SYNAPT XS for intact antibodies, a trastuzumab dilution series was analyzed by both denaturing and native LC-MS using an ACQUITY UPLC I-Class PLUS System and SYNAPT XS Mass Spectrometer with standard ESI source. The ACQUITY UPLC I-Class PLUS System was configured with a BEH C4 130 Å 1.7 μm, 2.1 x 50 mm Column with a water/acetonitrile/formic acid gradient for denaturing LC-MS analysis and a Protein BEH 200 Å SEC 2.1 x 150 mm Column with an isocratic 100 mM ammonium acetate elution for native LC-MS analysis. Additional LC-MS conditions are summarized in Figure 1. For denatured and native analysis, trastuzumab injections ranged from 2.5–50 ng and 0.1–2 μg, respectively. Lock mass data was collected during each acquisition and the correction was applied during post acquisition data processing via MassLynx Software v4.1.
Combined raw spectra for each of the dilution series of trastuzumab in denaturing and native analysis are displayed in Figures 2 and 3, respectively. Clean resolved spectra are observed in all sample amounts injected – down to 2.5 ng for denaturing analysis and 0.1 μg for native analysis. Peak valleys between first and second glycoforms were observed at 20–30% of the base peak, providing for accurate mass deconvolution by MaxEnt1 processing. A representative deconvoluted spectrum is displayed in Figure 4. In all samples, deconvoluted masses for the major glycoforms were less than 25 ppm compared to the calculated mass.
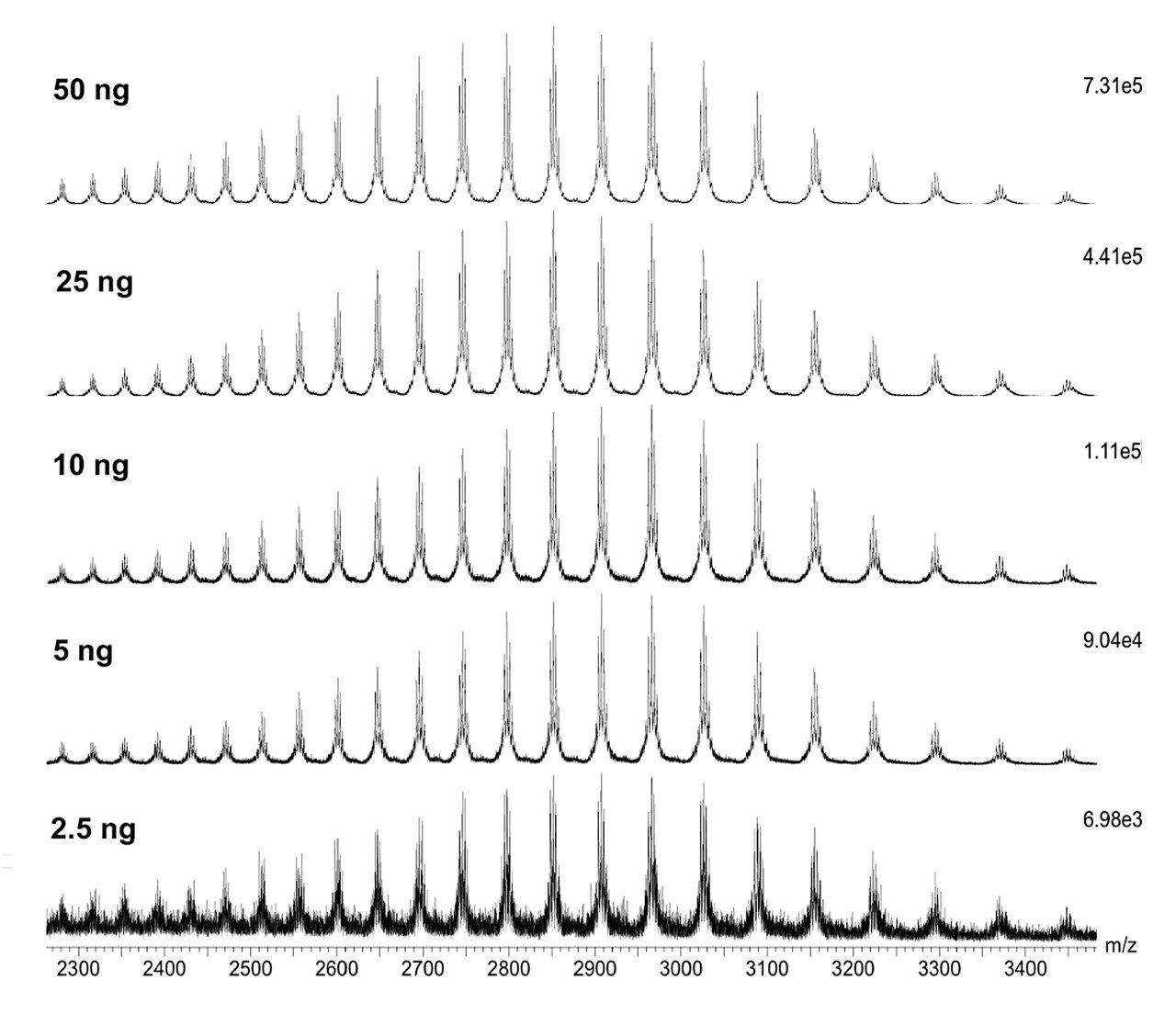
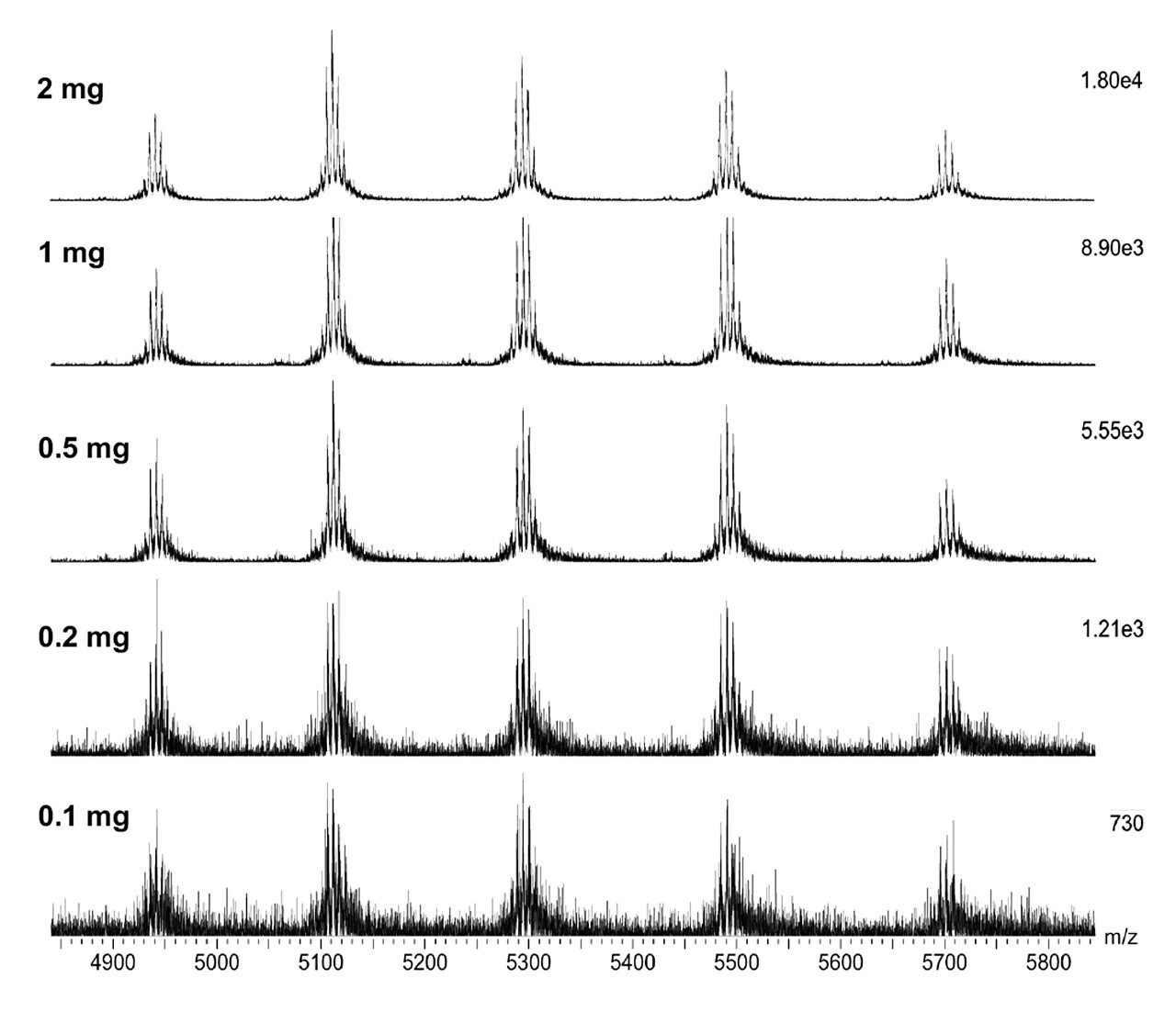
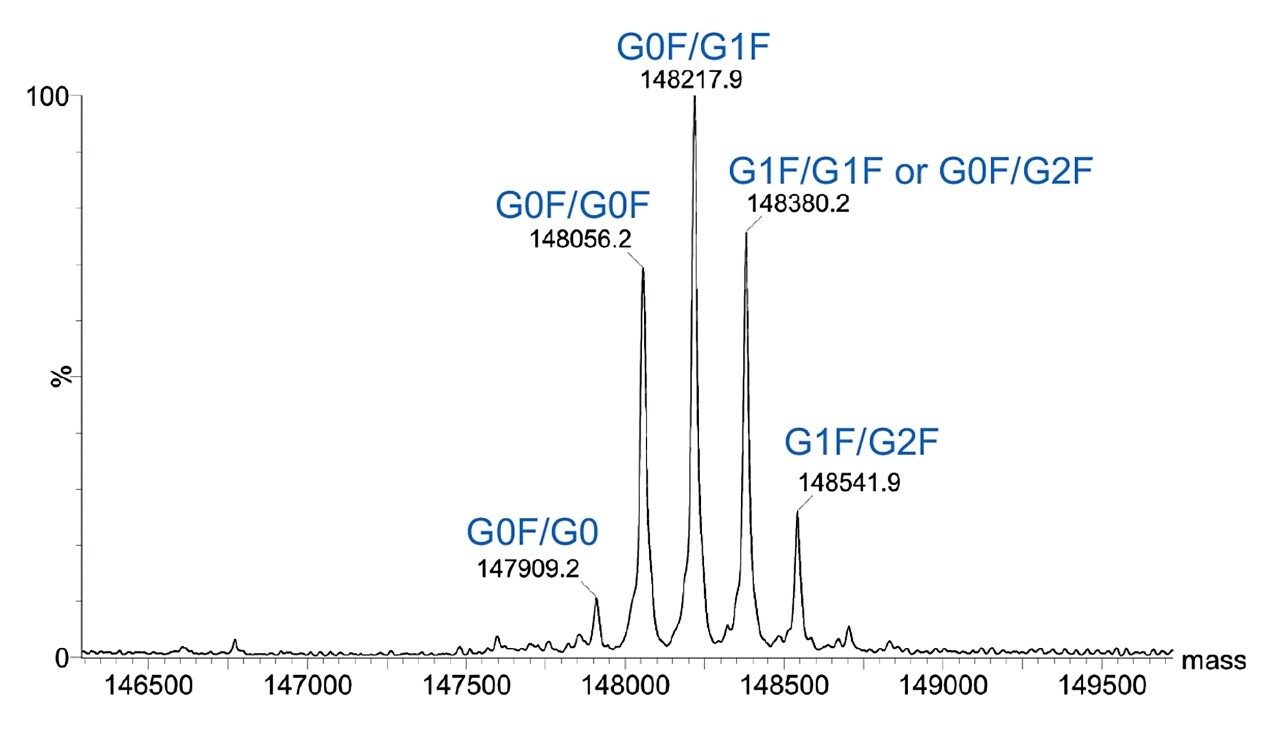
The SYNAPT XS, coupled with analytical scale chromatography, has provided high-quality data for all samples analyzed in this experiment – 2.5 ng for denaturing and 0.1 μg for native analysis, which could easily be deconvoluted by MaxEnt1 for accurate and sensitive characterization of intact trastuzumab. It is to be expected that the limit of detection for this type of analysis is even below the lowest amounts injected here. Improved resolution of the instrument allows for accurate intact mass analysis even at these low levels.
720006753, January 2020