
This is an Application Brief and does not contain a detailed Experimental section.
As a result of the ongoing COVID-19 pandemic, numerous small molecule drugs are being re-investigated as potential therapies to the novel coronavirus. Hydroxychloroquine, long prescribed as an anti-malarial, is a small molecule drug receiving an extended look. As recently as May 14th, 2020, the National Institute of Allergy and Infectious Diseases (NIAID) in the United States began a clinical trial for hydroxychloroquine in adults with mild to moderate COVID-19.1 To support analytical characterization during therapeutic development, this application brief offers a modernized MS compatible method for hydroxychloroquine sulfate analysis. Mass spectroscopy (MS) allows the investigator to accurately identify new or unknown components that may develop during the formulation process or routine testing. The new method offers higher resolution, less tailing, and faster run times compared to the current USP Monograph method.
Hydroxychloroquine (HQ) has long been prescribed for chemprophylaxis against malaria and, more recently, to help in the treatment of chronic autoimmune diseases.2 Interestingly, in vitro studies have shown that this active pharmaceutical ingredient might inhibit SARS-CoV-2 infection.3 It might be that HQ, like chloroquine, affects the glycosylation of angiotensin converting enzyme 2 (ACE2) and thereby hinders the novel coronavirus from entering cells.4 As a result, the NIAID in the United States began a Phase 2b clinical trial of hydroxychloroquine together with azithromycin for the treatment of mild to moderate COVID-19 in adults.1
In this application brief we present a modern, improved method for the assay of hydroxychloroquine sulfate in tablets with use of MS compatible buffer, while meeting USP system suitability requirements.
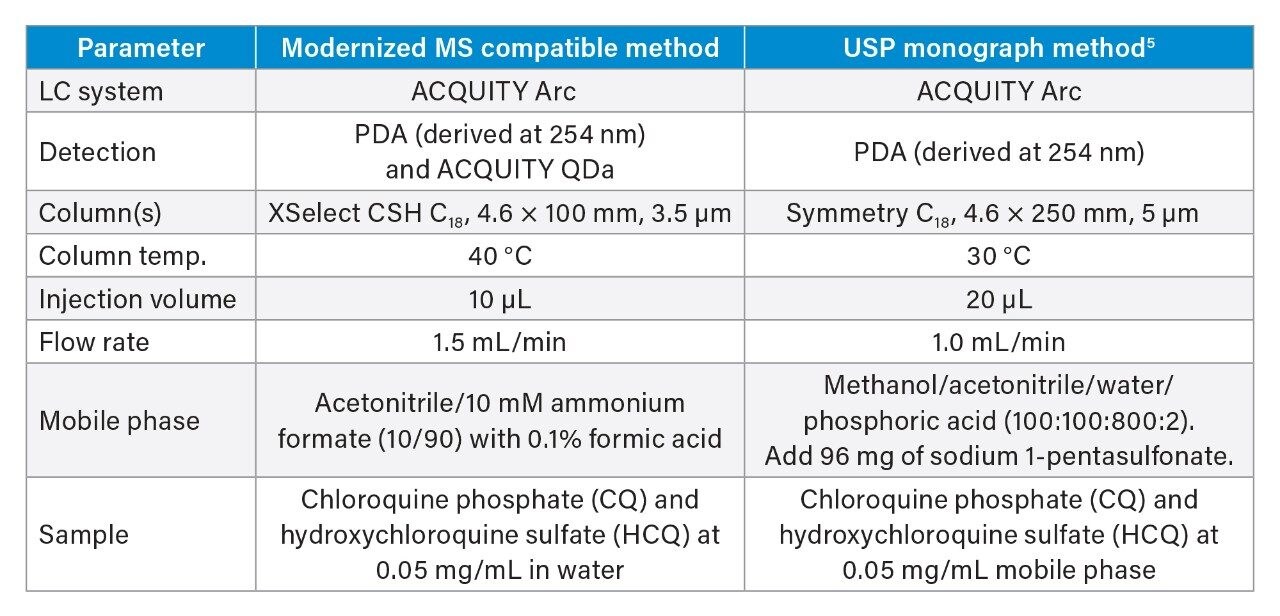
Conditions for the modernized MS compatible and current USP methods for the assay of hydroxychloroquine sulfate in tablets are summarized in Table 1.
Analysis of the system suitability solution performed using the new method resulted in a higher resolution between hydroxychloroquine and chloroquine peaks compared to the current USP method (Figure 2). Furthermore, faster run time was achieved with the improved method (3 minutes) compared to the USP method (7 minutes). The mass spectral data from the ACQUITY QDa Detector enabled quick and accurate peak identification by mass detection (Figure 3).
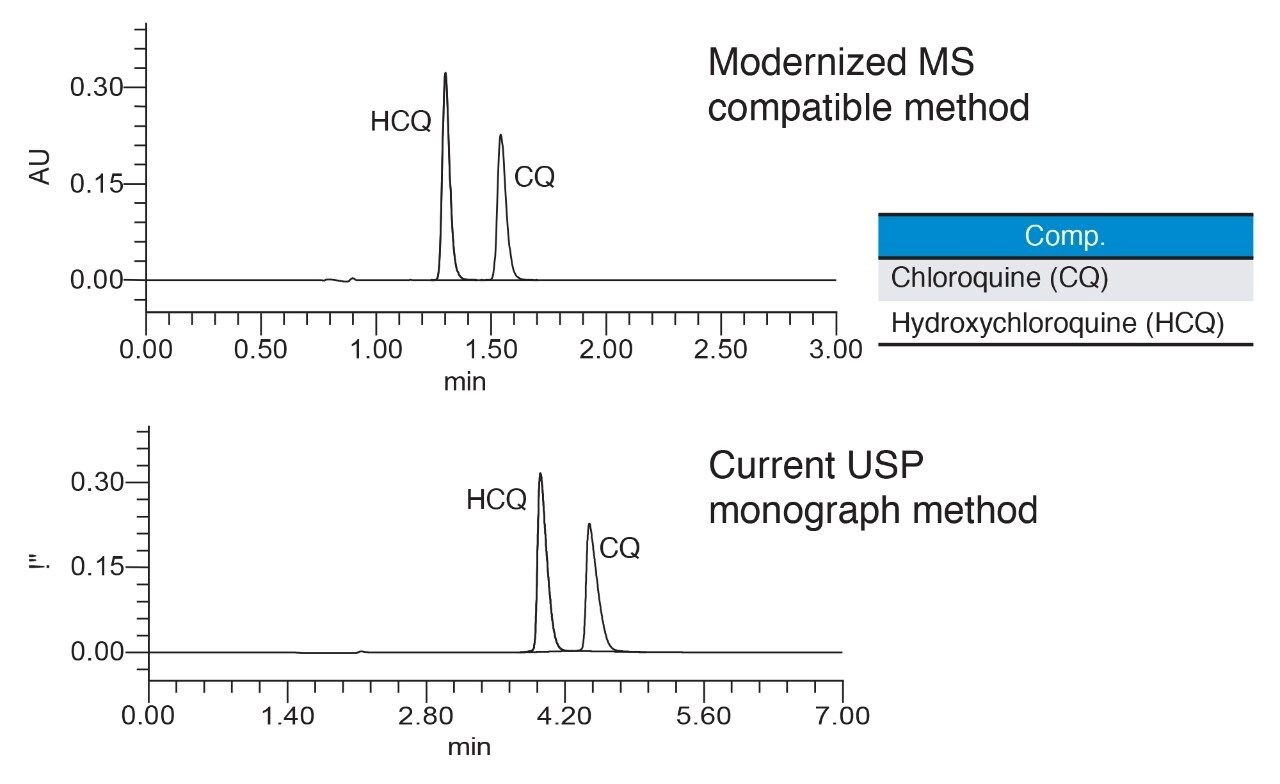
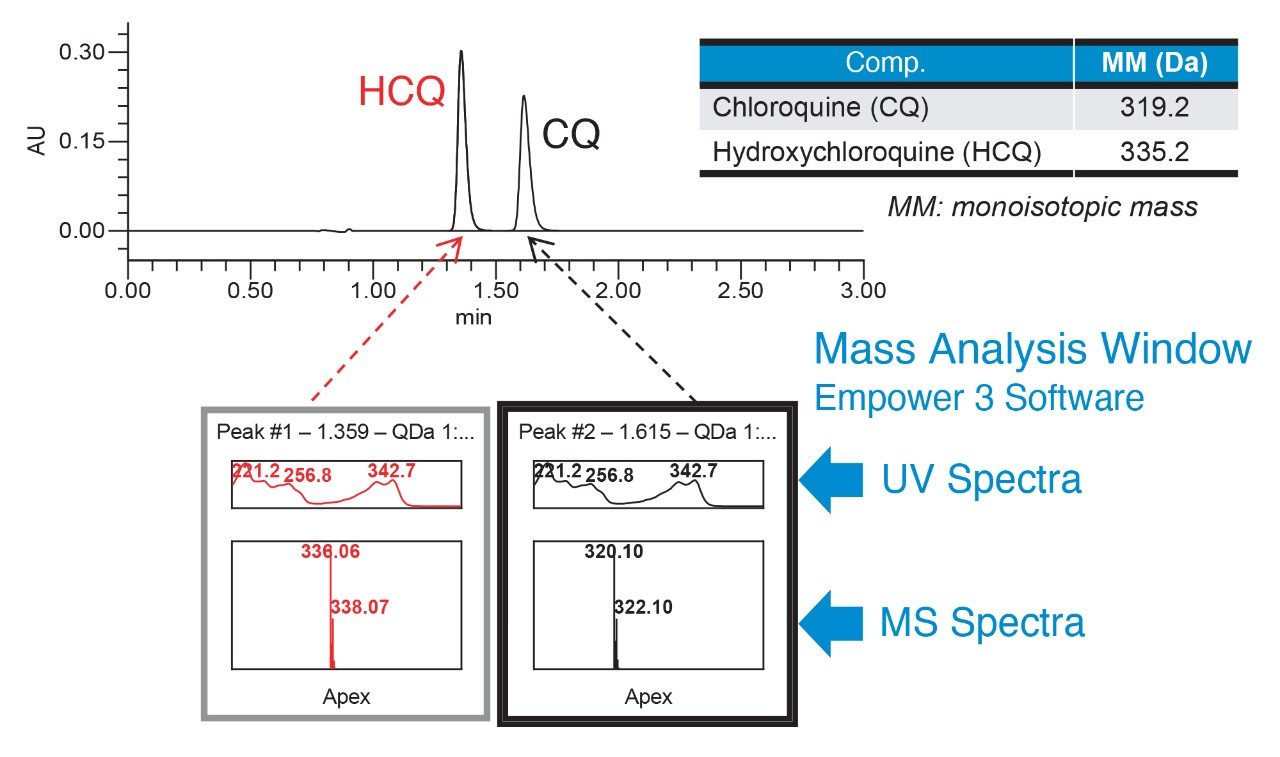
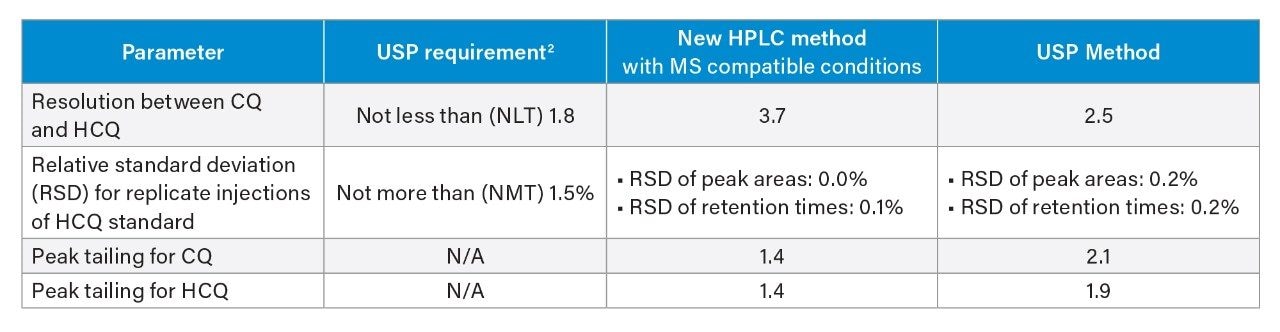
Performance of the modernized MS compatible method was measured against the system suitability requirements defined in the USP monograph for the assay of hydroxychloroquine sulfate in tablets5. The results showed that system suitability for the modernized MS compatible method successfully met the USP criteria (Table 2). Resolution between hydroxychloroquine and chloroquine improved significantly when run under modernized MS compatible conditions compared to the USP method. Relative standard deviations of peak areas and retention times for 5 replicate injections of the standard solution were less than 0.1%. Furthermore, the modernized MS compatible method provided improvements in USP tailing for both hydroxychloroquine and chloroquine.
This application brief provides an MS compatible method for assay of hydroxychloroquine sulfate in tablet formulation. By enabling MS analysis, this method enhances the analytical tool-kit available for hydroxychloroquine characterization and development. MS analysis enables qualitative compound identification without the need for individual standards. Furthermore, this modernized MS compatible method exhibits faster run time, improved resolution, and less peak tailing compared to the current USP Monograph method. As hydroxychloroquine is investigated as a potential treatment for COVID-19, improved speed prove to be important during time-sensitive drug development and subsequent manufacturing. Furthermore, more robust analytical performance may provide enhanced confidence in critical development and quality control environments during the novel coronavirus outbreak.
720006917, May 2020