
The ACQUITY UPLC I-Class System with the Xevo TQ-S Mass Spectrometer provides excellent sensitivity for detection, identification, and quantification of paralytic shellfish toxins and tetrodotoxin in shellfish tissues. This application note reports the results of the rapid, single-dispersive extraction, graphitized carbon-SPE HILIC-MS/MS method, validated for the determination of PSTs and TTX in shellfish.
A simple, rapid, cost-effective method for the determination of hydrophilic marine biotoxins in shellfish tissue samples, which meets the requirements for highly sensitive, accurate, precise, and reproducible testing at concentrations <1.5% of the EU maximum permitted limit.
Paralytic shellfish toxins (PST) are a naturally-occurring family of marine biotoxins, termed saxitoxins, produced by certain species of algae and bacteria, and reported globally. These algae are periodically found at high cell densities in the sea, during which they can accumulate in bivalve shellfish such as mussels, oysters, and clams.1 Tetrodotoxin (TTX) is another biotoxin, thought to be produced by marine bacteria, which has been found to accumulate in the tissues of shellfish.2 These toxins are potent neurotoxins, so may give rise to paralytic shellfish poisoning (PSP) in human consumers of contaminated products, resulting in the need for routine official control testing and end product testing of bivalve mollusks. In Europe, Regulation (EC) No. 854/2004, which has become part of Regulation (EC) 2017/625 as part of the revision of official control provision, requires a monitoring program of classified shellfish production areas to be established as part of the competent authority’s official controls to check for the possible presence of marine biotoxins in the shellfish flesh.3 All food business operators are required to ensure that they only place on the market food that is both safe and compliant with relevant requirements.
Both commercial and regulatory testing laboratories have interest in the need for a method that is simple, highly sensitive in relation to limits set in many countries, quick to use, and provides accurate, precise, and reproducible results. Most regulations around the world set maximum permitted levels (MPL) for PSP toxins as a group, typically 800 μg STX eq/kg of shellfish meat. Regulatory limits for marine biotoxins in shellfish in Europe are laid down in Regulation (EC) No. 853/2004.4
Within the European Union (EU), the official reference method for PST is AOAC OMA 2005.06 based on a pre-column oxidation liquid chromatography (LC) with fluorescence detection.5 While this provides an excellent level of regulatory control, the method is complex and time-consuming, requiring multiple clean-ups, derivatizations and analytical runs per sample, so a rapid one-shot method of analysis for PST is desirable.
The LC-FLD method is also unable to detect TTX, which has been found in shellfish throughout Europe in recent years. There is consequently great interest in the application of a recently developed method involving hydrophilic interaction liquid chromatography with tandem mass spectrometric detection (HILIC-MS/MS).6 HILIC-MS/MS utilizing ultra-performance liquid chromatography (UPLC) has been validated for PST7 and TTX8 and is currently undergoing further validation through collaborative study.
This application note reports the results of the rapid, single-dispersive extraction, graphitized carbon-SPE HILIC-MS/MS method, validated for the determination of PSTs and TTX in shellfish.
Homogenized shellfish tissues were extracted by mixing a sample with 1% acetic acid and heating it in a water bath. After cooling and further mixing, extracts were centrifuged before an aliquot of supernatant was neutralized and cleanup was performed using SPE with carbon cartridges. Extracts were mixed prior to dilution in polypropylene autosampler vials with MeCN. The details of the method are summarized in Figure 1.
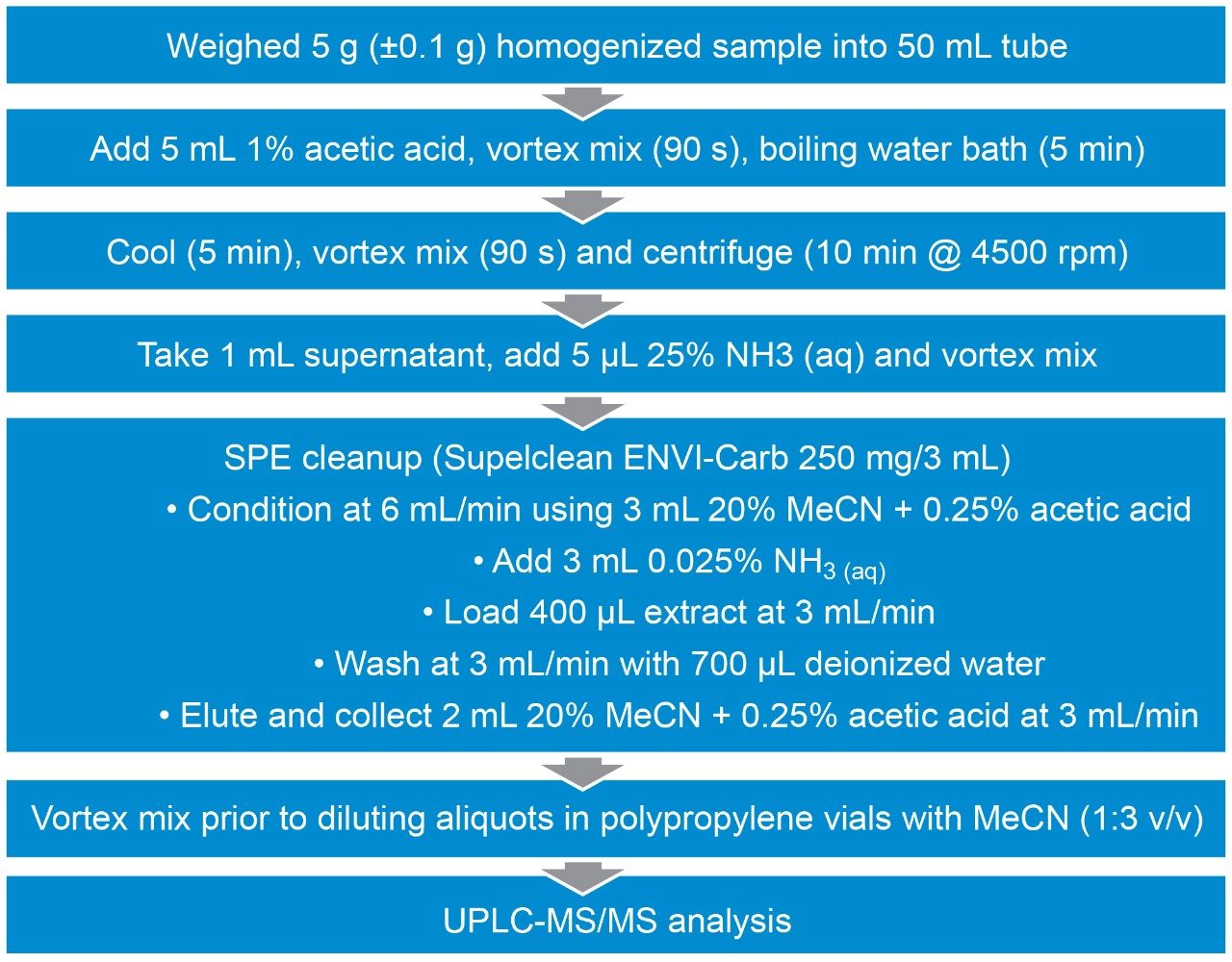
Primary toxin standards for PSP toxins and TTX were purchased from either the Institute of Biotoxin Metrology at the National Research Council, Canada or Cifga, Spain. Certified standards included STX di-HCl, NEO, GTX1&4, GTX2&3, GTX5, dcSTX, dcNEO, dcGTX2&3, C1&2, GTX6, and TTX. A mixed stock solution was prepared at relevant proportions with the transfer of equal volumes for each of the analogues. The mixed stock solution was then used to prepare a minimum of six matrix-matched calibration standards in blank mussel extract for external calibration quantitation.
|
UPLC system: |
ACQUITY UPLC I-Class with FTN Sample Manager |
|
Column: |
ACQUITY UPLC BEH Amide, 1.7 μm, 2.1 × 150 mm (p/n: 186004802) |
|
Guard column: |
ACQUITY UPLC BEH Amide 1.7 μm, 2.1 × 5 mm VanGuard Pre-column (p/n: 186004799) |
|
Mobile phase A1: |
Water/formic acid/NH3 (aq) (e.g. 500 mL+0.075 mL+0.3 ml) |
|
Mobile phase B1: |
Acetonitrile/water/formic acid (e.g. 700 mL+300 mL+0.1 ml) |
|
Solvent A2: |
Water/formic acid (e.g. 200 mL+1 ml) |
|
Solvent B2: |
Methanol |
|
Injection volume: |
2 μL |
|
Column temp.: |
60 °C |
|
Sample temp.: |
4 °C |
|
Run time: |
11 min |
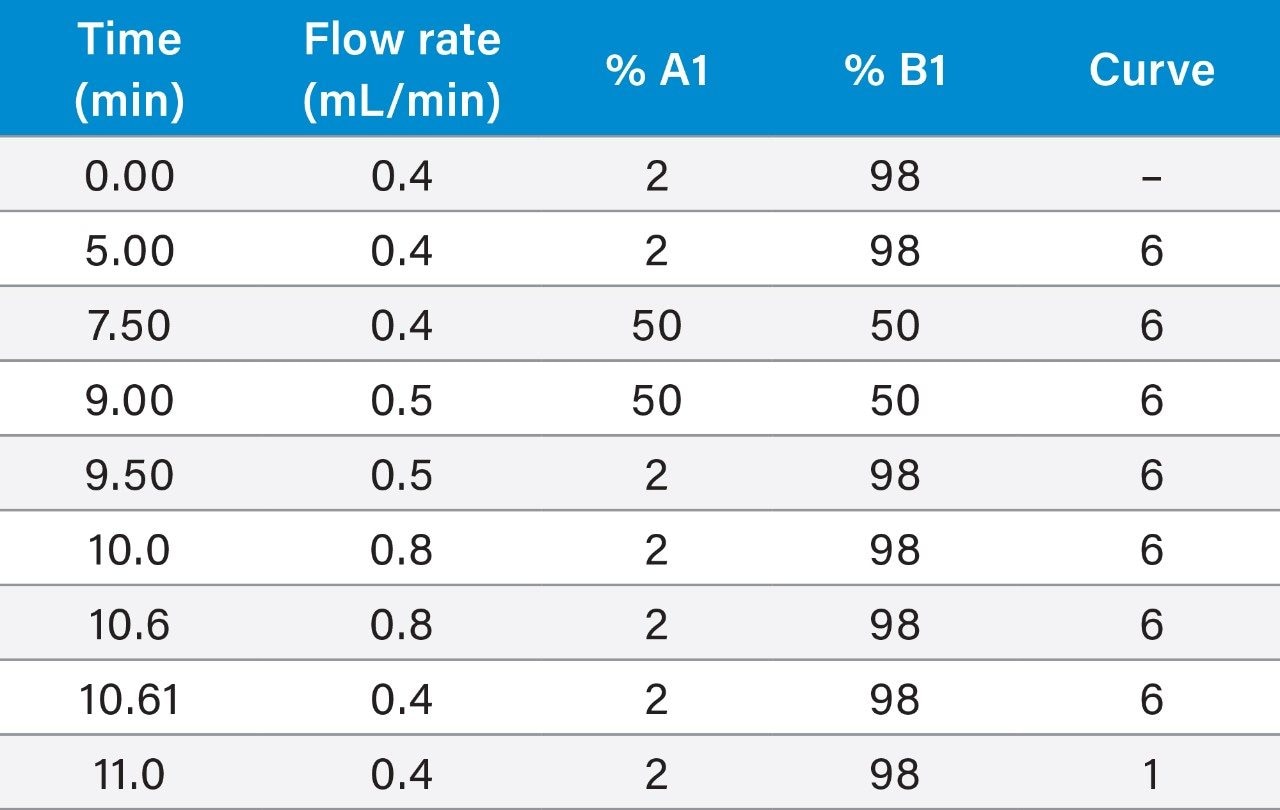
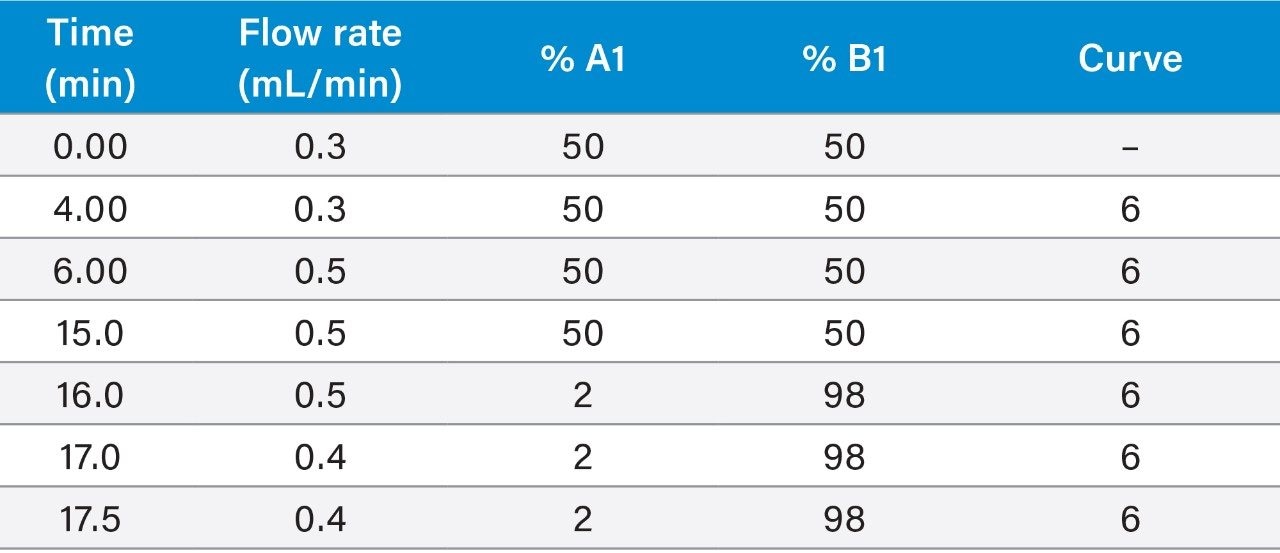
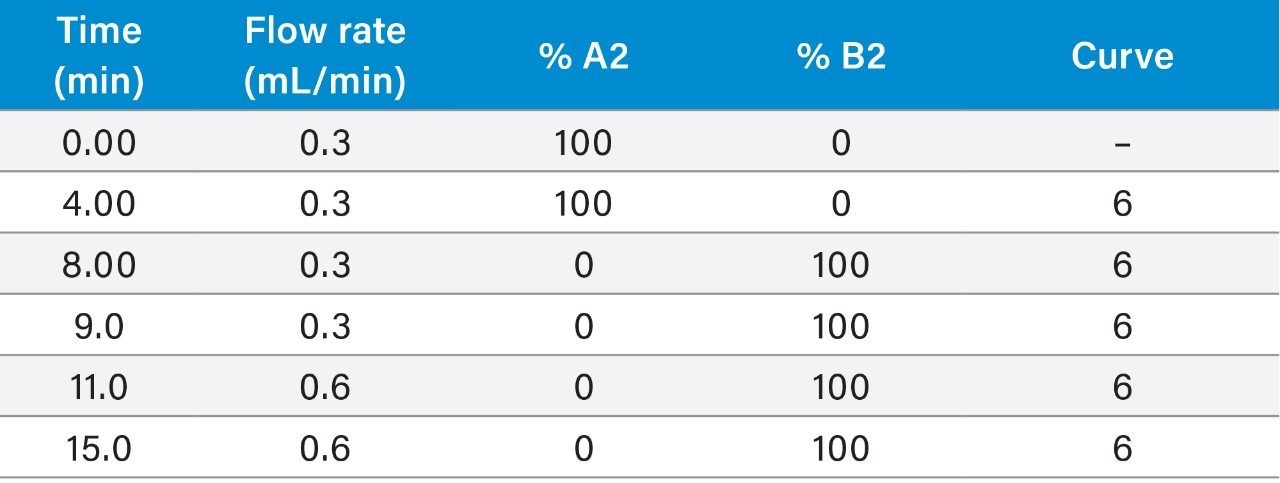
Although this method has been successfully validated using a gradient program containing changes in flow rate, some users might prefer to use 0.4 mL/min throughout. Ensure that enough time is provided to allow the column to re-equilibrate between injections. The column was conditioned prior to the analysis of each batch and flushed after use (see appendices for details).
|
MS instrument: |
Xevo TQ-S |
|
Source: |
Electrospray |
|
Polarity: |
Positive and negative ion mode |
|
Capillary voltage: |
+0.5 kV and -2.5 kV |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas flow: |
1000 L/Hr |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/Hr |
|
Cone voltage: |
10 V |
|
Nebulizer gas flow: |
7 Bar |
|
Collision gas flow: |
0.15 mL/min |
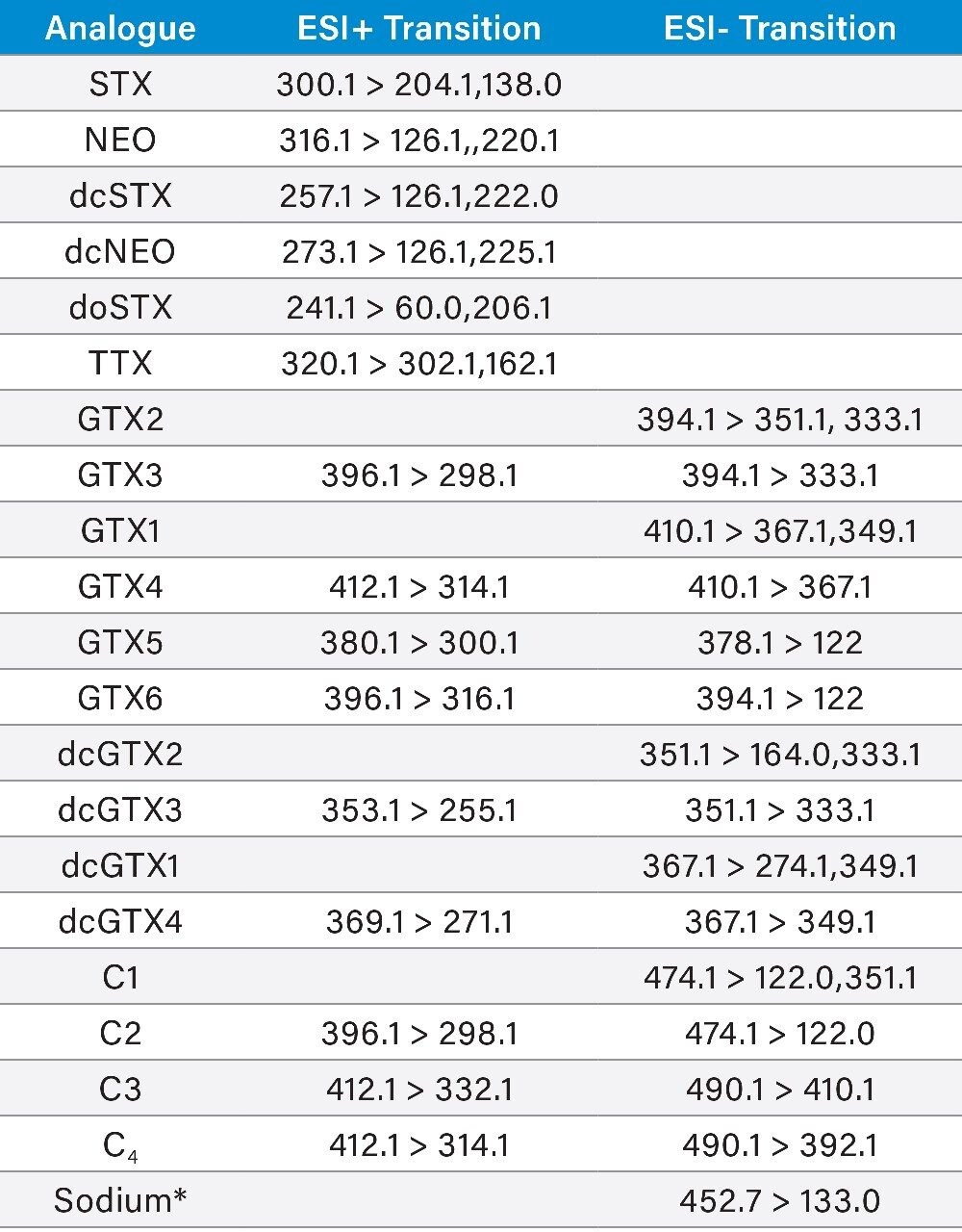
MS/MS acquisition methods, consisting of both positive and negative mode transitions are summarized in Table 1. Two MRM transitions that showed the best selectivity were used for each of the analytes. Recommended primary (quantitative) MRMs are indicated in bold in the table below. Sodium (as formate clusters) can be monitored using the selected ESI- transitions and provides an excellent indication of chromatographic separation of salts from the early eluting C toxins. The data were acquired using MassLynx Software v4.1 and processed using TargetLynx XS Application Manager. The optimum dwell time was set automatically using the AutoDwell function based upon a minimum of 12 data points per peak.
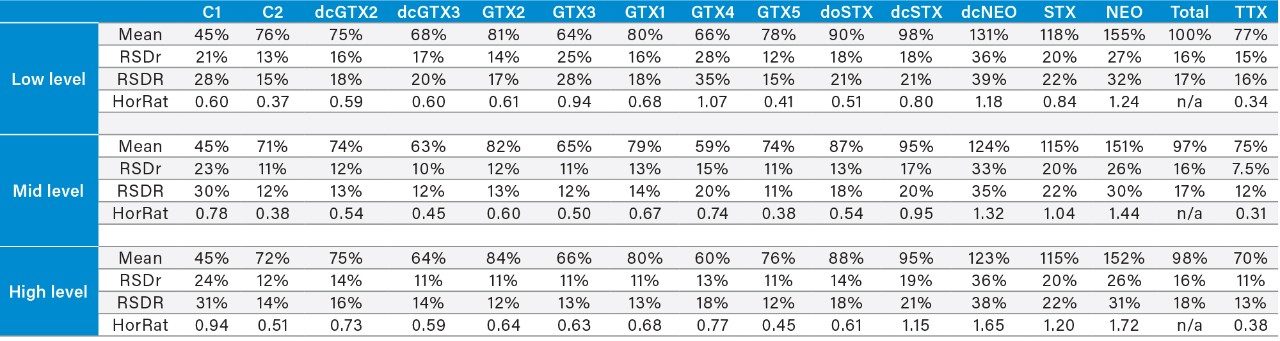
Within-laboratory method validation should be performed to provide evidence that a method is fit for the purpose for which it is to be used. To meet the requirements for use within official control regulatory testing, the performance of the method was assessed through the repeat analysis of spiked shellfish samples and naturally incurred reference materials. Twelve PST-free shellfish samples were sourced from both the UK and New Zealand, incorporating a variety of species of mussels, oysters, clams, cockles, and scallops. These were all used for both spiking studies and specificity determination for PST.7 For TTX method performance, validation was performed using mussels and oysters only.8 Method accuracy was assessed through the repeat analysis (n = 32; three months) of an oyster tissue CRM containing known concentrations of PST analogues. Toxin recovery, precision, and repeatability were determined in each matrix at low-, medium-, and high-toxin concentrations through replicate analysis of spiked sample extracts over multiple days (Table 2).7,8

Long-term within-laboratory reproducibility was calculated following the repeat analysis of a positive control laboratory reference material (LRM) over a period of five months. Sensitivity of the method as expressed by the limits of detection and quantitation (LOD and LOQ) were calculated from the signal-to-noise ratios (S/N) calculated from recovery data. Specifically, LOD and LOQ equated to the mean concentrations giving rise to primary MRM peaks with S/N of 3 and 10 respectively. The limit of reporting (LOR) was calculated as the concentration rounded up to the nearest significant figure, giving a S/N of 10 for the primary MRM and a S/N of three for the secondary MRM.7
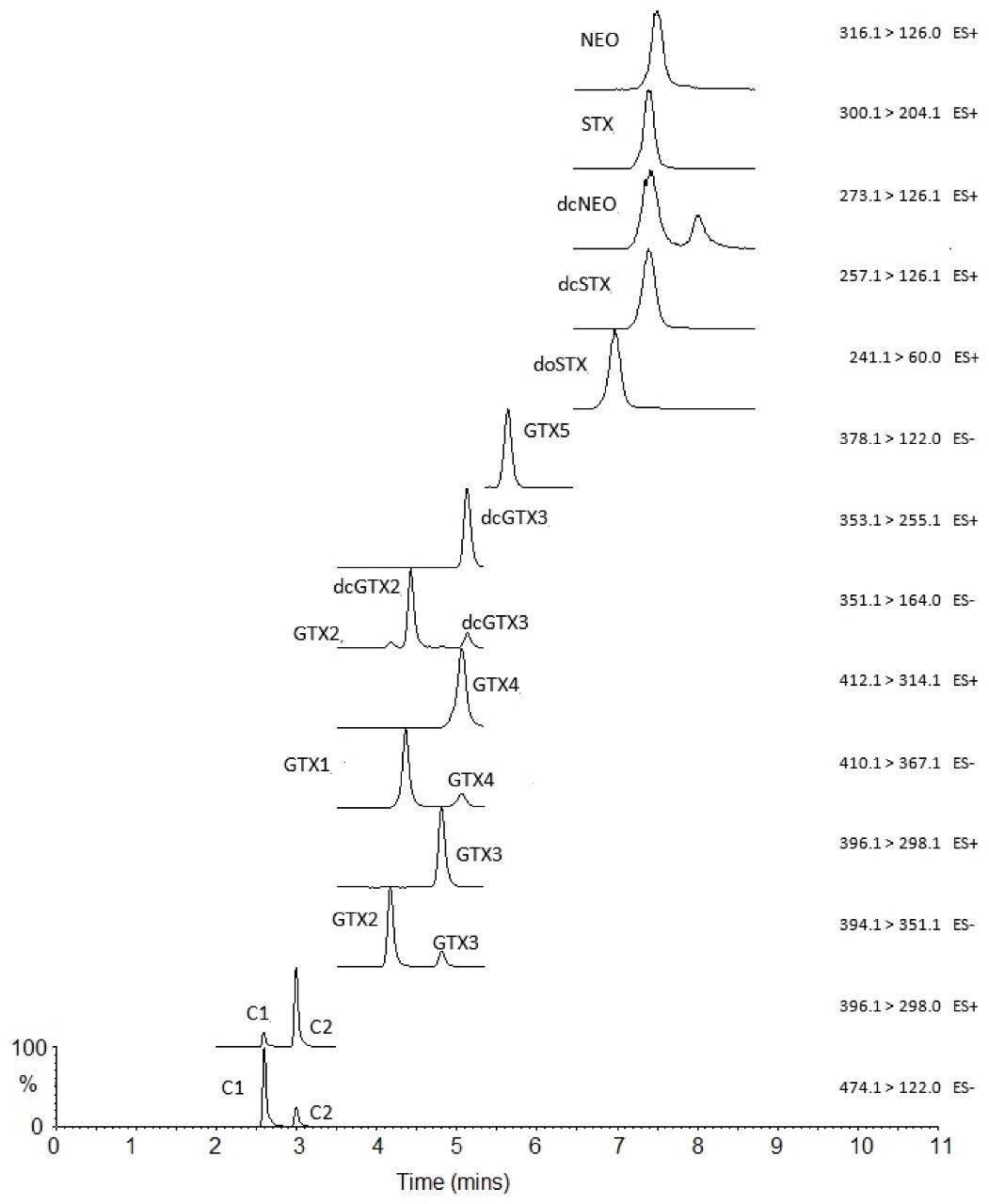
Validation of the method demonstrated excellent performance for the identification and quantitation of PST analogues and TTX in the 12 shellfish matrices studied. Figure 2 illustrates the separation and detection of all analytes achieved through use of the HILIC-MS/MS method following the analysis of a high-level calibration standard. Important considerations include the separation of each epimeric pair, as well as the separation of parent gonyautoxins from dicarbamoyl analogues, given the later fragment to the parent gonyautoxins in-source. Data from the analysis of PST-free shellfish extracts demonstrated the absence of chromatographic peaks with the same MRM and retention time characteristics as the PSP analytes. The linearity of the matrix-matched calibration standards was found to be excellent (r2<0.996) over the calibration range required.
Excellent sensitivity was demonstrated by the response for the MRM peaks detected from the analysis of spikes. Table 3 shows the mean LOD, LOQ, and LORs calculated across all shellfish matrices with low results demonstrating the suitability of the method for checking compliance against the EU MPL of 800 μg STX eq/kg for PST and the potential for screening and quantification at much lower concentrations (<1.5% of MPL per analyte) with the majority of analogues quantifiable at concentrations <10 μg STX eq/kg. For TTX, LOD/Q was <1.0 μg/kg, with the method LOR rounded up to 2.0 μg/kg, thereby demonstrating good sensitivity in comparison with the EFSA guidance limit of 44 μg/kg.9

Accuracy of the method was showed to be acceptable for selected PST analogues over the long-term with the repeat analysis of the PST matrix CRM over a two-year period (n = 45). Mean percentage recoveries for the analogues present in the CRM ranged from 85–127% with a mean total PST concentration of 646 μg STX eq/kg, equating to a mean recovery of total PSTs of 97% (Table 4).
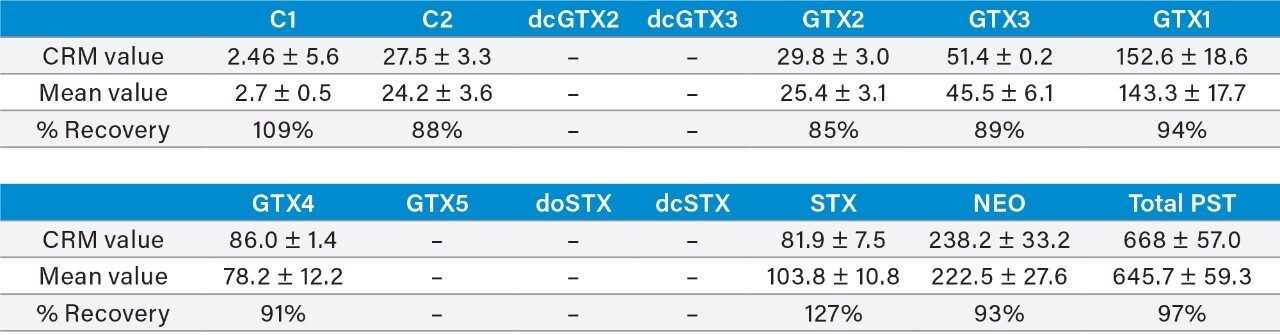
Method recovery is summarized in Table 5 for the three concentration levels examined. Recoveries were found to be similar across all concentrations, with the mean total PST concentration recovery ranging from 97–100% across all shellfish matrices. Most recoveries for individual PST analogues and TTX were between 65–125%, with a few exceptions. The mean within-batch and between-batch repeatability for the 12 matrices are also summarized in Table 5. Variabilities were consistent between the three concentration levels, with mean between-batch repeatability resulting in acceptable HorRat values with all <2.0, with the majority <1.0. Overall the results demonstrated acceptable recovery and repeatability of the method for most analytes.
The ACQUITY UPLC I-Class System with the Xevo TQ-S Mass Spectrometer provides excellent sensitivity for detection, identification, and quantification of paralytic shellfish toxins and tetrodotoxin in shellfish tissues. The method can be used for both screening and confirmation for the purpose of shellfish food safety testing. A simple single-step extraction of shellfish tissues in weak acetic acid prior to graphitized carbon solid phase extraction provided good recoveries for the compounds of interest and effective removal of co-extractives and salts, the source of significant matrix suppression during the analysis of shellfish by mass spectrometry. The Xevo TQ-S exhibited excellent sensitivity and robustness, but this same method could easily be transferred to other Xevo tandem quadrupole MS/MS instruments to meet the same performance criteria or extracts diluted further prior to UPLC-MS/MS.
720006709, October 2019